Abstract
Tuning the salinity and concentration of potential-determining ions, such as Mg2+, Ca2+, and SO42−, could alter the wettability toward a more water-wet state. The rate of alteration in carbonate rock wettability is a critical parameter to design the duration of the ion-engineered water flooding. Characteristic experiments, such as dynamic contact angle and pH measurements, ion chromatography, and spontaneous imbibition, are applied to study the rate of wettability alteration using different samples of ion-engineered water. Our study shows that the Caspian Sea water (CSW) with a salinity of 15,000 ppm is an efficient displacing fluid as it can initiate the multi-ion exchange (MIE) mechanism and alter the wettability from 86° to 35° within 2 d. The adjustment of salinity and active ion concentration makes the MIE mechanism much faster. For example, with five times diluted CSW, the same change in wettability is only achieved only within 9 h. Spiking the concentration of Ca2+ and SO42− ions is used to further shift the contact angle to 22° within 9 h. Spontaneous imbibition tests demonstrate that the rate of oil production doubles as a result of the ion-engineered brine due to the faster MIE process. The results obtained from this research work suggest that even a short period of interaction with optimized engineered water can affect the brine, oil, and carbonates interactions and change the reservoir rock initial wettability from neutral to strongly water-wet state. This allows to efficiently design engineered water flooding based on CSW in the field scale and make such projects more profitable.
Similar content being viewed by others
Introduction
The availability of formation brine, its nonnegative impact on the reservoir, ease of injection, and small capital and operational costs make water flooding projects attractive to be applied for many years as secondary recovery methods. The development of low-salinity water flooding (LSW) methods by altering the salinity of the injected water became attractive for carbonates when the first unexpected high oil recovery from the chalk reservoir at the Ekofisk field in the North Sea was produced (Al-Shalabi and Sepehrnoori 2016). Another term frequently used in LSW methods is ion-engineered brine, a brine in which the ion composition is changed by adding different salts.
One of the best examples of utilizing low-salinity water was a study carried out by Webb et al. (2005). They observed a reduction in residual oil saturation from 0.14 to 0.10 by increasing the concentration of sulfate ions in the injection brine. In 2007, Zhang et al. (2007) studied wettability alteration based on the performance of spontaneous imbibition curves by adding different ions. As a result of their work, SO42−, Mg2+, and Ca2+ were found to be potential-determining ions (PDIs), i.e., the main contributors to the wettability alteration toward the more water-wet state. It was suggested that to improve oil recovery, sulfate ions should act together with either calcium or magnesium cations. Moreover, it was found that the oil recovery is a strong function of temperature and is increased at higher temperatures. However, at high temperature, Mg2+ ions have a superior effect over Ca2+ (Zhang et al. 2007). Later, Gupta et al. (2011) studied ion-engineered brines with a low salinity content in the tertiary recovery mode by conducting core flooding experiments using dolomite and limestone cores. They observed incremental 9% and 5.1% oil recovery with spiked concentration of sulfate in the dolomite and limestone cores, respectively. Other laboratory studies and field trials verify the positive effect of low salinity and the importance of brine chemistry on oil recovery. Different studies have shown that for each crude oil/brine/rock (CBR) combination, there are an optimum salinity concentration and certain ion composition that could result in the most optimum incremental oil recovery (Al-Shalabi and Sepehrnoori 2016; Webb et al. 2005; Zhang et al. 2007; Gupta et al. 2011; Honarvar et al. 2020; Hiorth et al. 2010; Rashid et al. 2015; Rezaeidoust et al. 2009; Su et al. 2019; Strand et al. 2003; Austad et al. 2015; den Ouden et al. 2015; Zhang and Austad 2006; Zhang et al. 2006; Al-Kharusi et al. 2018; Fani et al. 2019; Yousef et al. 2012; Al-Attar et al. 2013; Alameri et al. 2014; Ayirala et al. 2019; Awolayo et al. 2014; Fathi et al. 2010; Ghandi et al. 2019; Jabbar et al. 2013; Jadhunandan and Morrow 1995; Karimi et al. 2016; Lashkarbolooki et al. 2017; Ligthelm et al. 2009; Robertson 2009; Tang and Morrow 1999; Tweheyo et al. 2006; Winoto et al. 2012; Yousef et al. 2010, 2011; Yu et al. 2008; Zaeri et al. 2018; Zahid et al. 2012; http://portal.kazntu.kz/files/publicate/2015).
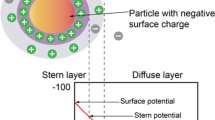
Different mechanisms are suggested to explain the additional oil recovery by LSW and ion-engineered brines in carbonates, with multi-ion exchange (MIE) and rock dissolution mechanisms the most-widely accepted (Honarvar et al. 2020; Hiorth et al. 2010; Rashid et al. 2015; Rezaeidoust et al. 2009; Su et al. 2019; Strand et al. 2003; Austad et al. 2015; den Ouden et al. 2015; Zhang and Austad 2006). By introducing low-salinity water into a CBR system, the initial conditions in the CBR are disturbed, which affects wettability. This alteration is due to the changes in surface potential by the adsorption of active ions due to their higher affinity and the desorption of organic material, known as the MIE process. The effects of ions, such as sulfate, calcium, and magnesium ions, on the activation of the MIE process have been studied by different researchers. It has been concluded that the simultaneous presence of sulfate with calcium or magnesium ions can enhance MIE and change the initial rock wettability (Strand et al. 2003; Zhang et al. 2006; Al-Kharusi et al. 2018). Another suggested mechanism of incremental oil recovery is rock dissolution proposed by Hiorth et al. (2010). They suggested two possible mechanisms to change the rock surface wettability as the sulfate adsorption onto the carbonate rock surface due to its higher affinity and calcite mineral dissolution to establish new wetting conditions in the system.
Wettability alteration is a key reason behind incremental oil recoveries in low-salinity water flooding projects. Attempts have been made to optimize the wettability alteration process as the main mechanism of LSW methods to make oil production much faster. As an example, a shift in wettability of a rock surface from 90° to 0° is reported to occur within 2 d by spiking the magnesium and sulfate ions in the ion-engineered injection brine (Fani et al. 2019). The ion-engineered brine injection duration is also critical to achieve the highest oil recovery. It was shown that the injection of 0.75 PV of ion-engineered brine was enough to achieve the highest wettability alteration and as a result the highest oil recovery (Yousef et al. 2012). These two works not only illustrated the role of PDI during tertiary flooding but also demonstrated how the process can be optimized to reach a faster wettability alteration.
The Caspian Sea region contains a large volume of oil and natural gas from both offshore and onshore deposits and is considered as a strategic and important region. All large known oil fields of Kazakhstan are located in this region. No comprehensive study has been carried out to assess the application of Caspian Sea water (CSW) as a low-salinity injection brine. Although CSW is considered as a low-salinity water with a total dissolved solids (TDS) of 15,000 ppm, by adjusting its chemical composition, it is possible to achieve higher oil recovery. In this study, the effect of active ions in CSW on the initiation of the MIE mechanism and the change in the wettability alteration rate are studied by different experiments, such as spontaneous imbibition tests, ion chromatography analysis, and pH and dynamic contact angle measurements. The MIE mechanism rate and consequently the oil production rate are improved by designing an appropriate ion-engineered brine.
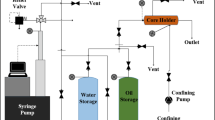
Methodology and materials
Rock and fluids
Core slices employed for contact angle measurements were cut from carbonate outcrops with similar properties to the formations in the region under study with an average permeability of 180 mD and a porosity in the range of 16–18%. The X-ray diffraction (XRD) analysis made using a Rigaku SmartLab automated X-ray diffractometer reveals that the samples were dominantly made of calcite. Core slices were cut in a semicircle shape with a radius of 4–5 cm and a height of 1 cm.
Dead heavy oil samples from an oil field in West Kazakhstan were collected, filtered through a 5-µm filter paper, and separated from water and any mechanical impurities before application in our tests. The viscosity and density of the oil were measured using viscometer from Anton Paar at different temperatures, as presented in Table 1. The gas chromatography–mass spectrometry method was used to determine the crude composition, as shown in Table 2.
High-salinity formation brine was prepared based on the chemical composition of the oil field in West Kazakhstan to represent carbonate fields in the Caspian Sea region (Akhmedzhanov et al. 2012). CSW was employed as the low-salinity brine. Ion composition analysis of the CSW was conducted using a Metrohm 930 Compact IC Flex ion chromatographer. Low-salinity brine samples were prepared by diluting CSW, while ion-engineered brines were prepared by adding different salts into five times diluted CSW. Ion concentrations were calculated based on the stoichiometry and molecular mass of the compounds. Table 3 shows the composition of all brines prepared by dilution of the CSW and the adjustment of active ion composition.
Core preparation
Core slices were cut from a carbonate outcrop and polished to have a smooth surface. They were then saturated with the formation brine at 80 °C for 2 h in a laboratory oven to set up the initial fluid distribution. The core slices were then aged in oil at 80 °C for 6 weeks in the laboratory oven to establish the initial wettability conditions of the reservoir. This time is required to reach a neutral wetting state. As at this wetting state the highest recovery factor during smart water flooding in carbonates is achieved (Rashid et al. 2015). Core plugs from the same formation were used for spontaneous imbibition tests, and they were cut to a 3-inch length. The porosity was measured using a helium porosimeter. The cores were then saturated with formation brine under an 1800 psi pressure for 1.5 d to establish the initial wetting conditions. The absolute permeability of the cores was then calculated according to Darcy’s law by continuous injection of formation brine at different flow rates. At each rate, the pressure drop across the core was recorded. This was carried out to make sure that the core was fully saturated with brine. The cores were then flooded with crude oil at different flow rates in the core flooding equipment until irreducible water saturation (\( S_{\text{wi}} \)) was reached. Finally, saturated cores were placed into oven for 6 weeks at 80 °C for the aging procedure. All measured parameters of the core plugs are presented in Table 4.
Contact angle measurements
After aging in oil, the slices were cleaned with a paper to remove excess oil from the surface. Finally, plates were soaked in different LSW and ion-engineered brine solutions at 80 °C for different time intervals of 3, 6, 9, 24, and 48 h. Contact angles were measured using an OCA 15EC video-based optical instrument. All aging procedures and laboratory experiments were performed at 80 °C, as this is the average temperature of reservoirs located in the Caspian Sea region (Al-Nofli et al. 2018). Contact angle measurements were used to observe the wettability alteration by different ion-engineered solutions. The angle between an oil drop and a rock surface was captured by taking a photo and analyzing it in SCA software version 4.5.14 Build 1064. The angle was measured by defining a baseline at the solid interface and drawing the tangent line to the oil drop contour. The instrument measures the left- and right-side readings of the droplet. All the measurements were taken three times and the average value reported.
Ion chromatography analysis
The ion chromatography instrument (Metrohm 930 Compact IC Flex-Ion Chromatographer) was utilized to identify the ion composition of the brines. Measurements were taken at the same time intervals as the contact angle experiments. The maximum allowed salinity the equipment can tolerate is 4000 ppm. For this reason, all brines were diluted 100 times with deionized water before the measurement.
Spontaneous imbibition
Spontaneous imbibition experiments were carried out to test the potential change of rock wettability by introducing ion-engineered water. In this study, imbibition tests were performed as a secondary recovery process. For this purpose, two aged carbonate core plugs were placed into two separate Amott cells from VINCI Technologies and then placed in an oven at 80 °C for spontaneous imbibition tests. The first test was conducted in a cell full of the most effective ion-engineered brine while the second core was in a cell filled with CSW. The oil recoveries by spontaneous imbibition in these two cells were measured with time and compared with each other to analyze the effect of ion management on the performance of the brine in altering the rock wettability. The oil recovery factor was calculated as a percentage of OOIP.
Results and discussion
Effect of dilution on contact angle measurements
The main aim of the contact angle measurement was to design the most optimum composition of active ions and dilution for altering the rock wettability to the more water-wet state. This design was performed in a two-stage procedure. First, the effect of dilution was studied and the most effective diluted CSW was selected. In the second stage, the active ions in the best diluted sample were adjusted to improve the performance of the final brine. For the first stage, after aging for 1.5 months in crude oil, the contact angle of the rock samples was measured and showed a neutral wetting state. All core slices were then categorized into five groups and placed in five different brines, namely, deionized (DI) water, CSW, five times diluted CSW, ten times diluted CSW, and 20 times diluted CSW. The contact angle of an oil drop was measured after 3, 6, 9, 27, and 48 h. Figure 1 shows the dynamic wettability alteration of a carbonate rock surface for the five tested brines.
From Fig. 1, it can be seen that the application of DI water did not result in a noticeable angle change due to the lack of active ions and therefore cannot be applied as a displacing fluid, in agreement with (Awolayo et al. 2014). In contrast, when immersed in CSW, five times diluted, and ten times diluted brines, the wettability alteration process initiated during the first 3 h of the interaction, but it took 9 h in the case of 20 times diluted CSW. This observation proves the requirement of the presence of a minimum concentration of PDIs to activate the wettability alteration process. Therefore, it can be concluded that to have an immediate response of the carbonate rock to the injection of LSW, the minimum salt concentration should be more than 850 ppm, i.e., the salt concentration of 20 times diluted CSW. Secondly, introducing the CSW into the CBR system shows significant results. The CSW was able to shift the initial contact angle from 86° to 41° in 2 d. Moreover, the dilution of CSW five times and ten times further decreased the angle to 34° in 2 d. The 20 times diluted CSW was also able to alter the initial wettability of the carbonate rock surface, but only to 51°. Thus, there is an optimum dilution ratio and ion concentration, which result in the optimum contact angle alteration. The trend for the contact angle change and ion concentration is plotted in Fig. 2 for different time intervals.
Figure 2 shows that brines with a high salt concentration are not efficient in contact angle adjustments, due to the presence of a high number of inactive ions not participating in the alteration process, such as Na+ and Cl−, which prevent active ions from approaching the surface. In addition, very diluted fluids containing a small amount of PDIs are not efficient in modifying the wettability state of the system and did not result in any contact angle change, Thus, there is an optimum salt concentration that is required to have the highest contact angle shift, in addition to a certain concentration range that could activate the wettability alteration process. As can be seen in Fig. 2, at all time intervals, increasing the TDS concentration from 0 to 3000 ppm resulted in an increase in the CA change. However, for brine with a TDS of 15,000 ppm at the same time intervals, a much lower CA alteration was observed.
The rate of wettability alteration in all cases was also different. Figure 3a shows the early time contact angle change. In all brine solutions, the angle between the oil and rock surface progressively increases, indicating the detachment of an oil droplet and changing the carbonate surface to the more water-wet state. In contrast, there is no positive increase in contact angle during the late time analysis, as shown in Fig. 3b, due to the system stabilization. Since the objective of the current work was to find the most optimal brine solution that can have a maximum wettability alteration of the surface in the shortest possible time, five times diluted CSW was chosen as the basis to study the ion effect.
Effect of PDI management on contact angle
For the second stage, five different solutions were prepared to examine the effect of PDIs (Mg2+, Ca2+, and SO42− ions) on the rate of wettability alteration and to analyze the possibility of achieving a faster change in the contact angle by ion management. In all samples, the total dissolved solid amount was kept approximately the same, in the range of 5000–5500 ppm. To investigate the effect of PDIs, the concentration of different ions was spiked five to seven times. For example, in 5X_Mg brine, the concentration of magnesium ion was increased fivefold, in 5X_Ca brine, the concentration of calcium ion was increased sevenfold, and in the 5X_Mg_SO4 and 5X_Ca_SO4 brines, the concentration of sulfate ions was increased simultaneously with calcium or magnesium ions to analyze the effect of counter ions.
Cores slices were placed into the containers with brine, following the same procedure as discussed previously in the first stage. Contact angles were measured after 3, 6, and 9 h. Figure 4 shows the results of CA as a function of time. To distinguish the impact of a certain ion, the results are compared with 5X brine. No more improvement was observed by application of the 5X_SO4 and 5X_Mg_SO4 brines. In contrast, for the 5X_Mg, 5X_Ca, and 5X_Ca_SO4 brines, a further decrease in CA was observed. Moreover, the rate of the wettability alteration process for the 5X_Mg, 5X_Ca, and 5X_Ca_SO4 brines was higher. For example, for the 5X brine, a contact angle of 35° was achieved within 9 h, but it took only 6 h for the 5X_Mg, 5X_Ca, and 5X_Ca_SO4 brines to reach the same change in CA. The presence of only sulfate ions is not enough to alter the initial wettability, as can be seen from Fig. 4. The 5X_SO4 brine shows the same performance as the five times diluted brine. Hence, the presence of active cations is also required, which proves the catalytic behavior of the sulfate ion in the process. In our case, due to the temperature, calcium is more active for wettability alteration than magnesium.
The same trend as previously observed was found while studying the effect of dilution. All brine solutions showed their highest CA changes during the first 9 h of the experiment. Aging for a longer time did not result in a greater CA modification.
The early time alteration in contact angle for the five times diluted CSW with a spiked concentration of Ca2+ and SO42− simultaneously is shown in Fig. 5 as the most effective case. The highest change in CA from 86° to 22° was observed. The combination of cation and anion PDIs was able to decrease the contact angle by an additional 12° compared to the 5X diluted CSW. Hence, the 5X_Ca_SO4 brine was selected for further spontaneous imbibition experiments.
Ion chromatography analysis
In this study, ion chromatography analysis was used to provide evidence and support regarding the underlying mechanism of wettability alteration by ion-engineered brines in carbonates. Brine samples from the core slice aging bottle were collected at different times and analyzed to understand the distribution of ions during the MIE and the wettability alteration mechanism. The results of the geochemical properties of these samples for the best ion-engineered case (5X_Ca_SO4) are shown in Fig. 6a, b as examples. The dashed lines correspond to the initial ion concentrations.
In 5X_Ca_SO4, the ions stabilize after 9 h in contact with the CBR system. This indicates that the ion exchange mechanism happened during this time interval, in agreement with the CA results, where the maximum possible shift happened during the first 9 h (Fig. 7). For this reason, in the discussion of ion exchange, only the early time period is considered. As mentioned in the Methodology and materials section, core slices were first aged in the formation brine, which does not contain sulfate ions. An illustration of the initial ion distribution is presented in Fig. 8a. As can be seen in Fig. 6a, at the early stage of the process and once the rock is in contact with the ion-engineered 5X_Ca_SO4 brine, a decrease in sulfate concentration occurs due to the higher composition of sulfate in the engineered brine compared to the formation brine. Hence, adhesion of sulfate ions to the rock surface occurs, as schematically shown in Fig. 8b. As a result, the negatively charged oil components are released and dissociated in water according to reactions (1) and (2), while \( {\text{SO}}_{4}^{2 - } \) remains stuck to the carbonate rock surface.
Finally, oil components are adsorbed by Ca2+ ions and released from the rock to the surrounding brine as \( \left( {\text{RCOO}} \right)_{2} {\text{Ca}} \) through reaction (3), which is schematically shown in Fig. 8c. To compensate for the decrease in calcium ions due to their release through \( \left( {\text{RCOO}} \right)_{2} {\text{Ca}} \), most likely carbonate dissolution took place, as shown in reaction 4. Carbonate dissolution produces calcium ions, yielding its overall increase, which can be observed in Fig. 6a, where the calcium ion concentration is increased during the first 9 h. By rock dissolution, the rock surface becomes more negatively charged, which leads to more repulsion of the oil component.
The ion-engineered fluid injected into the CBR system had a much lower salinity concentration compared to the formation brine. Therefore, to finally attain a chemical balance in the system, there was a substantial increase in \( {\text{Na}}^{ + } \) and \( {\text{Cl}}^{ - } \) ions due to the movement of these ions from the formation brine on the rock to the injection brine, as shown in Fig. 6b. The proposed MIE mechanism is in agreement with the procedure suggested in Zhang et al. (2006) and Al-Kharusi et al. (2018).
The Mg2+ concentration did not change during the whole experiment, which shows that this cation did not participate in the MIE process and is not active. This finding is in agreement with the work of Su et al. (2019), where they showed that the effect of Ca2+ on the wettability alteration is greater than that of Mg2+ at temperatures in the range of our study.
The same trend of ion distribution as in 5X_Ca_SO4 was observed in 5X brine, proof that the multi-ion exchange mechanism took place. However, the initial amount and consumptions of \( {\text{SO}}_{4}^{ - 2} \) and \( {\text{Ca}}^{ + 2} \) were much lower than in 5X_Ca_SO4 case. As a result, the rate and extent of wettability alteration were lower. Same as previously, \( {\text{Na}}^{ + } \) and \( {\text{Cl}}^{ - } \) ions concentration increased, indicating an exchange with formation brine to reach chemical equilibrium.
pH analysis
The aim of the pH analysis during aging by 5X_Ca_SO4 brine was to confirm the mechanism involved in the oil recovery process. A plot of pH versus time is presented in Fig. 9. Generally, the brine showed a decrease in pH, which is likely due to the washing of acidic crude oil components into the brine. The same trend was observed during aging in 5X brine. There are two possible mechanisms to describe this change in the pH as the surface ion exchange and CO2 production. As discussed previously, during surface ion exchange, described by reactions (1)–(3), the oil component dissociates and produces H+ ions. This mechanism was first proposed by Austad et al. (Zhang et al. 2006; Al-Kharusi et al. 2018). Due to the adsorption of sulfate ions into the carbonate rock surface, the oil component is released and dissociates in water by producing protons, indicating an acidic environment. Moreover, the pH decrease can be associated with CO2 production:
Spontaneous imbibition test
The aim of the SI tests was to compare the rate of wettability alteration and oil recovery by CSW and five times diluted CSW with spiked concentrations of \( {\text{Ca}}^{2 + } \) and \( {\text{SO}}_{4}^{2 - } \) ions as the optimum case. The tests were carried out at 80 °C. Core 1 was used for CSW, and core 2 was used for 5X_Ca_SO4. Figure 10 demonstrates oil expulsion during the SI experiment. The trend of recovery factory versus time is depicted in Fig. 11.
Due to the low permeability and heterogeneity of the core plugs and the high viscosity of oil, the recovery process occurred relatively slowly. For example, it took 30 h to reach a plateau in production for both cases. Even though the core with lower permeability was used with 5X_Ca_SO4 as an imbibing fluid, the higher and faster oil recovery was observed. The ultimate recovery factor (RF) by 5X_Ca_SO4 brine was 10.7%, whereas RF by CSW was only 5.5% in 2 d. The SI test results confirm the outcomes of CA measurements, which show the more shift to the water-wet state by injection of 5X_Ca_SO4 brine. The imbibition process is high at the beginning and declines when the rock becomes more water wet. PDIs are involved in the MIE process, as discussed previously. Expulsion of oil from the core is initiated by the adsorption of \( {\text{SO}}_{4}^{2 - } \), desorption of negatively charged oil components, making the rock surface more positively charged, and repulsion between the rock surface and active cations, which leads to detachment of oil droplets. In agreement with the contact angle measurements, the rate of oil expulsion is doubled when the rock is imbibed with 5X_Ca_SO4 brine compared to CSW.
The rates of oil recovery and alteration in CA are plotted in Fig. 12. The CA change per time was calculated for each brine. The average rate was 5.06°/h for CSW and 7.04°/h for the ion-engineered brine. As shown in Fig. 12, the oil recovery rate by CA followed the exact same rate as CA alteration, which confirms the governing mechanism of oil detachment.
These experiments and analysis show that by adjusting active ions of the injection brine, it is possible to achieve higher rate of wettability alteration and more oil recovery. This point is important specially for application in cases such as CSW, which is naturally a low-saline brine. Design of the ion-engineered brine improves the behavior of the flooding in such cases in carbonate formation.
Conclusions
The impact of the adjustment of PDIs (\( {\text{SO}}_{4}^{2 - } \), \( {\text{Ca}}^{2 + } \), and \( {\text{Mg}}^{2 + } \)) in Caspian Sea injection water to develop an optimized ion-engineered brine and water to affect the rate of wettability alteration in carbonate formations was studied. Studying both effects of salinity and ion types on the rate of wettability alteration can be considered as the novelty of this work. Caspian Sea water samples were used for this study as an important source in the region. The following items are concluded:
-
An optimum salt concentration is required to initiate the process of MIE and changes the wettability to the most water-wet state. For CSW, five times dilution brine showed the best effect by changing CA by more than 50°. The simultaneous presence of \( {\text{Ca}}^{2 + } \) and \( {\text{SO}}_{4}^{ - 2} \) ions could further change the contact angle and make the rock more water wet after 9 h of contact. Ion-engineered solutions affect CA during the first 9 h of the experiment. Aging for a longer time did not result in any more effective CA modifications. Five times diluted Caspian Sea water with a spiked concentration of \( {\text{Ca}}^{2 + } \) and \( {\text{SO}}_{4}^{ - 2} \) was the most effective solution to initiate MIE process.
-
Ion chromatography results reveal that the main mechanism behind ion-engineered water injection is MIE that requires adsorption of \( {\text{SO}}_{4}^{ - 2} \) ions and cations, as \( {\text{Ca}}^{2 + } \). Moreover, results have demonstrated that sulfate ions accelerate the wettability alteration process. The ion exchange occurred mainly during the first 9 h, which was in agreement with the change in CA.
-
It was possible to achieve 5.5% of OOIP recovery by Caspian Sea water and 10.7% by 5X_Ca_SO4 brine during SI tests. The higher rate of wettability alteration by the optimized ion-engineered water results in the faster oil production by SI.
References
Akhmedzhanov TK, Abd Elmaksoud AS, Baiseit DK, Igembaev IB (2012) Chemical properties of reservoirs, oil and gas of Kashagan field, southern part of pre-Caspian depression, Kazakhstan. Int J Chem Sci 10:568–578
Alameri W, Teklu TW, Graves RM, Kazemi H, AlSumaiti AM (2014) Wettability alteration during low-salinity water-flooding in carbonate reservoir cores. In: Society of Petroleum Engineers—SPE Asia Pacific oil and gas conference and exhibition, APOGCE 2014—changing the game: opportunities, challenges and solutions
Al-Attar HH, Mahmoud MY, Zekri AY, Almehaideb R, Ghannam M (2013) Low-salinity flooding in a selected carbonate reservoir: experimental approach. J Petrol Explor Prod Technol. https://doi.org/10.1007/s13202-013-0052-3
Al-Kharusi B, Pourafshary P, Mosavat N, Al-Wahaibi Y (2018) Design and performance of smart water shock injection SWSI in carbonate reservoirs. In: Society of Petroleum Engineers—SPE annual caspian technical conference and exhibition 2018, CTCE 2018. https://doi.org/10.2118/192557-MS
Al-Nofli K, Pourafshary P, Mosavat N, Shafiei A (2018) Effect of initial wettability on performance of smart water flooding in carbonate reservoirs: an experimental investigation with IOR implications. Energies 11(6):1394. https://doi.org/10.3390/en11061394
Al-Shalabi EW, Sepehrnoori K (2016) A comprehensive review of low salinity/engineered water injections and their applications in sandstone and carbonate rocks. J Petrol Sci Eng. https://doi.org/10.1016/j.petrol.2015.11.027
Austad T, Shariatpanahi SF, Strand S, Aksulu H, Puntervold T (2015) Low salinity EOR effects in limestone reservoir cores containing anhydrite: a discussion of the chemical mechanism. Energy Fuels. https://doi.org/10.1021/acs.energyfuels.5b01099
Awolayo A, Sarma H, AlSumaiti AM (2014) A laboratory study of ionic effect of smart water for enhancing oil recovery in carbonate reservoirs. In: Society of Petroleum Engineers—SPE EOR conference at oil and gas West Asia 2014: driving integrated and innovative EOR
Ayirala S, Saleh S, Enezi S, Yousef A (2019) Multiscale water ion interactions at interfaces for enhanced understanding of smartwater flooding in carbonates. In: International petroleum technology conference 2019, IPTC 2019. https://doi.org/10.2523/iptc-19066-ms
Composition of formation water in Kazakhstan oil fields (Online). http://portal.kazntu.kz/files/publicate/2015-12-21-elbib_2.pdf. Accessed 29 Mar 2020
den Ouden L, Nasralla RA, Guo H, Bruining H, van Kruijsdijk CPJW (2015) Calcite dissolution behaviour during low salinity water flooding in carbonate rock. In: IOR 2015—18th European symposium on improved oil recovery. https://doi.org/10.3997/2214-4609.201412102
Fani M, Al-Hadrami H, Pourafshary P, Vakili-Nezhaad G, Mosavat N (2019) Optimization of smart water flooding in carbonate reservoir. In: Society of Petroleum Engineers—Abu Dhabi international petroleum exhibition and conference 2018, ADIPEC 2018. https://doi.org/10.2118/193014-ms
Fathi SJ, Austad T, Strand S (2010) “Smart water” as a wettability modifier in chalk: the effect of salinity and ionic composition. Energy Fuels. https://doi.org/10.1021/ef901304m
Ghandi E, Parsaei R, Riazi M (2019) Enhancing the spontaneous imbibition rate of water in oil-wet dolomite rocks through boosting a wettability alteration process using carbonated smart brines. Petrol Sci. https://doi.org/10.1007/s12182-019-0355-1
Gupta R, Smith GG, Hu L, Willingham T, Lo Cascio M, Shyeh JJ, Harris CR (2011) Enhanced waterflood for carbonate reservoirs: impact of injection water composition. https://doi.org/10.2118/142668-ms
Hiorth A, Cathles LM, Madland MV (2010) The impact of pore water chemistry on carbonate surface charge and oil wettability. Transp Porous Media. https://doi.org/10.1007/s11242-010-9543-6
Honarvar B, Rahimi A, Safari M, Khajehahmadi S, Karimi M (2020) Smart water effects on a crude oil-brine-carbonate rock (CBR) system: further suggestions on mechanisms and conditions. J Mol Liq. https://doi.org/10.1016/j.molliq.2019.112173
Jabbar MY, Al-Hashim HS, Abdallah W (2013) Effect of brine composition on wettability alteration of carbonate rocks in the presence of polar compounds. In: Society of Petroleum Engineers—SPE Saudi Arabia section technical symposium and exhibition. https://doi.org/10.2118/168067-ms
Jadhunandan PP, Morrow NR (1995) Effect of wettability on waterflood recovery for crude-oil/brine/rock systems. SPE Reserv Eng (Soc Petrol Eng). https://doi.org/10.2118/22597-PA
Karimi M, Al-Maamari RS, Ayatollahi S, Mehranbod N (2016) Wettability alteration and oil recovery by spontaneous imbibition of low salinity brine into carbonates: impact of Mg2+, SO42− and cationic surfactant. J Petrol Sci Eng. https://doi.org/10.1016/j.petrol.2016.09.015
Lashkarbolooki M, Ayatollahi S, Riazi M (2017) Mechanistical study of effect of ions in smart water injection into carbonate oil reservoir. Process Saf Environ Prot. https://doi.org/10.1016/j.psep.2016.11.022
Ligthelm D, Gronsveld J, Hofman J, Brussee N, Marcelis F, Van Der Linde H (2009) Novel waterflooding strategy by manipulation of injection brine composition (SPE-119835). In: 71st European Association of Geoscientists and Engineers conference and exhibition 2009: balancing global resources. Incorporating SPE EUROPEC 2009
Rashid S, Mousapour MS, Ayatollahi S, Vossoughi M, Beigy AH (2015) Wettability alteration in carbonates during “smart waterflood”: underling mechanisms and the effect of individual ions. Colloids Surf A Physicochem Eng Asp 487:142–153. https://doi.org/10.1016/j.colsurfa.2015.09.067
Rezaeidoust A, Puntervold T, Strand S, Austad T (2009) Smart water as wettability modifier in carbonate and sandstone: a discussion of similarities/differences in the chemical mechanisms. Energy Fuels. https://doi.org/10.1021/ef900185q
Robertson EP (2009) Low-salinity waterflooding improves oil recovery—historical field evidence. JPT J Petrol Technol. https://doi.org/10.2118/0109-0047-jpt
Strand S, Standnes DC, Austad T (2003) Spontaneous imbibition of aqueous surfactant solutions into neutral to oil-wet carbonate cores: effects of brine salinity and composition. Energy Fuels. https://doi.org/10.1021/ef030051s
Su W, Liu Y, Yang H, Pi J, Chai R, Li C (2019) New insights into the mechanism of wettability alteration during low salinity water flooding in carbonate rocks. J Dispers Sci Technol. https://doi.org/10.1080/01932691.2018.1478306
Tang GQ, Morrow NR (1999) Influence of brine composition and fines migration on crude oil/brine/rock interactions and oil recovery. J Petrol Sci Eng. https://doi.org/10.1016/S0920-4105(99)00034-0
Tweheyo MT, Zhang P, Austad T (2006) The effects of temperature and potential determining ions present in seawater on oil recovery from fractured carbonates. In: Proceedings—SPE symposium on improved oil recovery
Webb KJ, Black CJJ, Tjetland G (2005) A laboratory study investigating methods for improving oil recovery in carbonates. In: International petroleum technology conference proceedings. https://doi.org/10.2523/iptc-10506-ms
Winoto W, Loahardjo N, Xie X, Yin P, Morrow NR (2012) Secondary and tertiary recovery of crude oil from outcrop and reservoir rocks by low salinity waterflooding. In: SPE—DOE improved oil recovery symposium proceedings. https://doi.org/10.2118/154209-ms
Yousef AA, Al-Saleh S, Al-Kaabi A, Al-Jawfi M (2010) Laboratory investigation of novel oil recovery method for carbonate reservoirs. In: Society of Petroleum Engineers—Canadian unconventional resources and international petroleum conference, vol 3, pp 1825–1859. https://doi.org/10.2118/137634-ms
Yousef AA, Al-Saleh S, Al-Jawfi M (2011) New recovery method for carbonate reservoirs through tuning the injection water salinity: smart water flooding. In: 73rd European Association of geoscientists and engineers conference and exhibition 2011: unconventional resources and the role of technology. Incorporating SPE EUROPEC 2011. https://doi.org/10.2118/143550-ms
Yousef AA, Liu JS, Blanchard GW, Al-Saleh S, Al-Zahrani T, Al-Zahrani RM, Al-Tammar HI, Al-Mulhim N (2012) Smart waterflooding: industry. https://doi.org/10.2118/159526-ms
Yu L, Evje S, Kleppe H, Kårstad T, Fjelde I, Skjaeveland SM (2008) Analysis of the wettability alteration process during seawater imbibition into preferentially oil-wet chalk cores. In: Proceedings—SPE symposium on improved oil recovery
Zaeri MR, Hashemi R, Shahverdi H, Sadeghi M (2018) Enhanced oil recovery from carbonate reservoirs by spontaneous imbibition of low salinity water. Petrol Sci. https://doi.org/10.1007/s12182-018-0234-1
Zahid A, Shapiro A, Skauge A (2012) Experimental studies of low salinity water flooding in carbonate reservoirs: A new promising approach. In: Society of Petroleum Engineers—SPE EOR conference at oil and gas West Asia 2012, OGWA–EOR: building towards sustainable growth
Zhang P, Austad T (2006) Wettability and oil recovery from carbonates: effects of temperature and potential determining ions. Colloids Surf A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2006.01.009
Zhang P, Tweheyo MT, Austad T (2006) Wettability alteration and improved oil recovery in chalk: the effect of calcium in the presence of sulfate. Energy Fuels. https://doi.org/10.1021/ef0600816
Zhang P, Tweheyo MT, Austad T (2007) Wettability alteration and improved oil recovery by spontaneous imbibition of seawater into chalk: impact of the potential determining ions Ca2+, Mg2+, and SO42−. Colloids Surf A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2006.12.058
Acknowledgements
The authors would like to thank Nazarbayev University for supporting this research through the NU Faculty Development Competitive Research Grants program (Grant Number 110119FD4541).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the co-authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bazhanova, M., Pourafshary, P. Impact of SO42−, Ca2+, and Mg2+ ions in Caspian Sea ion-engineered water on the rate of wettability alteration in carbonates. J Petrol Explor Prod Technol 10, 3281–3293 (2020). https://doi.org/10.1007/s13202-020-01006-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-020-01006-z