Abstract
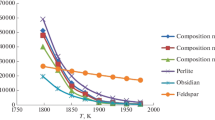
Measurements are performed and new data are obtained on the viscosities of the solutions formed by the inorganic ingredients of heat-insulating and slag-forming Izoterm-1600 materials based on volcanic silicon dioxide in a liquid phase in the temperature range 1525–1700°C. The influence of the total concentration of basic oxides FeO, MgO, CaO, K2O, and Na2O and the amphoteric Al2O3 oxide on the viscosity of the oxide solution is studied. The concentration–temperature change in the viscosity of the multicomponent oxide solution is described by linear and exponential laws. The obtained dependences make it possible to predict and calculate the viscosity in a low-temperature region. The dependence of the viscosity of the oxide solution on the total concentration of basic oxides and amphoteric Al2O3 is shown to be satisfactorily described by a linear law.


Similar content being viewed by others
REFERENCES
V. A. Kapitanov, A. V. Kuklev, E. G. Polozov, et al., “Heat-insulating properties of slag mixtures for a tundish,” Stal’, No. 1, 28–31 (2009).
V. A. Shablovsky, Yu. V. Klimov, N. F. Onishchenko, et al., “Specialized mixtures for siphon casting of steel,” Stal’, No. 6, 21–24 (2009).
S. K. Vil’danov and A. V. Likhodievskii, “Heat-insulating and protective mixture for a metal mirror in the tundish of a continuous casting machine,” RF Patent 2334587, Byull. Izobret., No. 27 (2008).
S. K. Vil’danov and A. V. Likhodievskii, “Method of thermal insulation of metal and slag when pouring steel into molds,” RF Patent 2410190, Byull. Izobret., No. 3 (2012).
S. K. Vil’danov, A. V. Likhodievskii, and A. N. Pyrikov, “Development and implementation of energy-saving materials for steel casting,” Novye Ogneupory, No. 8, 3–6 (2011).
S. K. Vil’danov, “Development and implementation of heat-insulating and slag-forming materials of the Izoterm-1600 series,” Stal’, No. 9, 17–22 (2018).
S. K. Vil’danov and V. S. Valavin, “Investigation of the properties of the slags formed during liquid-phase reduction of converter sludge,” Metally, No. 6, 23–28 (1993).
J. O’M. Bockris, J. D. Mackenzie, and J. A. Kitchener, “Viscous flow in silica and binary liquid silicates,” Trans. Faraday Soc. 51, 1734–1748 (1955).
O. V. Mazurin, M. V. Streltsina, and T. P. Shvaiko-Shvaikovskaya, Properties of Glasses and Glass-Forming Melts: A Handbook, Vol. IV, Part 1, One- and Two-Component Oxide Systems (Nauka, Leningrad, 1980).
M. S. Aslanova, V. A. Chernov, and L. F. Kulakov, “Viscosity and crystallization properties of quartz glass with alloying additions of aluminum oxide,” Steklo Keramika, No. 6, 19–21 (1974).
M. P. Volarovich and A. A. Leont’ev, “Determination of the viscosity of quartz glass in the softening area,” ZhFKh 8 (3), 335–338 (1936).
G. Hofmaier and G. Urbian, “The viscosity of pure silica,” Sci. Ceram. 4, 25–32 (1968).
J. F. Bacon, A. Kasapis, and J. Wholley, “Viscosity and density of molten silica and high silica content glasses,” Phys. Chem. Glass. 1 (3), 90–98 (1960).
R. Bruckner, “Properties and structure of vitreous silica,” J. Non-Cryst. Solids 5 (2–3), 123–216 (1970).
N. K. Gusakova, V. K. Leko, E. V. Meshcheryakova, and R. B. Lebedeva, “Viscosities of various quartz glasses over a wide temperature range,” Izv. Akad. Nauk, Ser. Neorg. Mater. 10 (2), 338–340 (1974).
V. K. Leko, “Joint influence of the impurities of the oxides of alkali metals and aluminum on the viscosity of vitreous silica,” Fiz. Khim. Stekla 6 (5), 553–557 (1980).
V. K. Leko, “Viscosity of quartz glasses,” Fiz. Khim. Stekla 5 (3), 258–278 (1979).
A. A. Varuzhanyan, “Viscosities of acidic volcanic glasses in the range of softening temperatures under the pressure of water vapor,” in Laws of the Formation and Placement of Volcanic Glass Deposits (Nauka, Moscow, 1969), pp. 160–169.
M. A. Bezborodov, Viscosity of Silicate Glasses (Nauka Tekhnika, Minsk, 1975).
L. V. Sheludyakov, Composition, Structure, and Viscosity of Homogeneous Silicate and Aluminosilicate Melts (Nauka, Alma-Ata, 1980).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by K. Shakhlevich
Rights and permissions
About this article
Cite this article
Vil’danov, S.K. Viscosity of the Oxide Solutions Based on Silicon Dioxide and Formed upon Melting of Heat-Insulating and Slag-Forming Izoterm-1600 Materials in Metallurgical Units. Russ. Metall. 2020, 1032–1037 (2020). https://doi.org/10.1134/S0036029520090153
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029520090153