Abstract
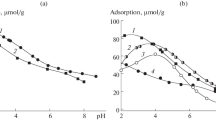
Adsorption isotherms are obtained for limonene enantiomers on the surfaces of ionol and adenine dinitrate crystals, and for the crystalline structures of cytosine and cyanuric acid deposited on surfaces of graphitized thermal carbon black. Viedma ripening conditions are used to induce chirality in the crystals. It is found that the isotherms of limonene enantiomers differ from one another during adsorption on all four samples. The shape of the adsorption isotherms also differs for cytosine and cyanuric acid, indicating different mechanisms of adsorption. It is assumed that one enantiomer is partially adsorbed in the cavity of the supramolecular structure of cytosine and cyanuric acid, while the second is not. The degree of surface filling at which different adsorption isotherms are observed shows that the stability of the layers of enantiomers on the topologically chiral surface of the crystals differs.





Similar content being viewed by others
REFERENCES
V. A. Davankov, Symmetry 10, 749 (2018).
V. Schurig, J. Chromatogr., A 906, 275 (2001).
E. Gil-Av, B. Feibush, and R. Charles-Sigler, Tetrahedron Lett. 7, 1009 (1966).
V. A. Davankov and S. V. Rogozhin, J. Chromatogr., A 60, 280 (1971).
S. V. Rogozhin and V. A. Davankov, J. Chem. Soc. D: Chem. Commun., No. 10, 490a (1971). https://doi.org/10.1039/C2971000490A
V. A. Davankov, Chirality 9, 99 (1997).
V. A. Davankov, Chromatografia 27, 475 (1989).
E. W. Meijer and A. R. A. Palmans, Angew. Chem. Int. Ed. 46, 8948 (2007).
M. Fujiki, Symmetry 6, 677 (2014).
A. G. d. Bruin, M. E. Barbour, and W. H. Briscoe, Polym. Int. 63, 165 (2014).
M. Liu, L. Zhang, and T. Wang, Chem. Rev. 115, 7304 (2015).
V. Percec and P. Leowanawat, Isr. J. Chem. 51, 1107 (2011).
R. E. Morris and X. Bu, Nat. Chem. 2, 353 (2010).
K.-H. Ernst, Orig. Life Evol. Biosphere 40, 41 (2010).
L. J. Prins, P. Timmerman, and D. N. Reinhoudt, J. Am. Chem. Soc. 123, 10153 (2001).
D. K. Kondepudi, R. J. Kaufman, and N. Singh, Science (Washington, DC, U. S.) 250, 975 (1990).
C. Viedma, Phys. Rev. Lett. 94, 065504 (2005).
L.-C. Sogutoglu, R. R. E. Steendam, H. Meekes, et al., Chem. Soc. Rev. 44, 6723 (2015).
D. K. Kondepudi, J. Digits, and K. Bullock, Chirality 7, 62 (1995).
F. C. Frank, Biochim. Biophys. Acta 11, 459 (1953).
W. L. Noorduin, H. Meekes, A. A. C. Bode, et al., Cryst. Growth Des. 8, 1675 (2008).
T. Kawasaki, K. Suzuki, Y. Hakoda, and K. Soai, Angew. Chem. 47, 496 (2008).
H. Mineki, T. Hanasaki, A. Matsumoto, et al., Chem. Commun. 48 (85), 10538 (2012).
T. Kawasaki, K. Suzuki, K. Hatase, et al., Chem. Commun., No. 17, 1869 (2006). https://doi.org/10.1039/b602442d
T. Kawasaki, M. Nakaoda, N. Kaito, et al., Orig. Life Evol. Biospheres 40, 65 (2010).
D. T. McLaughlin, T. P. T. Nguyen, L. Mengnjo, et al., Cryst. Growth Des. 14, 1067 (2014).
H.-M. Zhang, Z.-X. Xie, L.-S. Long, et al., J. Phys. Chem. C 112, 4209 (2008).
K. E. Plass, A. L. Grzesiak, and A. J. Matzger, Acc. Chem. Res. 40, 287 (2007).
V. Y. Gus’kov, D. A. Sukhareva, Y. Y. Gainullina, et al., Supramol. Chem. 30, 940 (2018).
V. Yu. Gus’kov, D. A. Sukhareva, I. V. Arslanova, and D. E. Musabirov, J. Anal. Chem. 72, 1089 (2017).
V. Y. Gus’kov, Y. Y. Gainullina, R. I. Musina, et al., Sep. Sci. Technol. 55 (2020, in press).
A. V. Kiselev and Ya. I. Yashin, Gas Adsorption Chromatography (Khimiya, Moscow, 1967) [in Russian].
F. Kh. Kudasheva, V. Yu. Gus’kov, and E. R. Valinurova, Adsorption. Theory and Practice (RITs BashGU, Ufa, 2014) [in Russian].
S. Gregg and K. Sing, Adsorption, Surface Area, and Porosity (Academic, New York, 1982).
D. L. Barker and R. E. Marsh, Acta Crystallogr. 17, 1581 (1964).
T. Wandlowski, D. Lampner, and S. M. Lindsay, J. Electroanal. Chem. 404, 215 (1996).
R. Otero, M. Lukas, R. E. A. Kelly, et al., Science (Washington, DC, U. S.) 319, 312 (2018).
M. Bolte and M. Amon, Experimental Crystal Structure Determination, CCDC 156796 (Cambridge Crystallogr. Data Centre, 2001).
Funding
This work was financially supported be the Russian Science Foundation, project no. 19-73-10079.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Tulyabaev
Rights and permissions
About this article
Cite this article
Guskov, V.Y., Ramazanova, G.A., Allayarova, D.A. et al. Adsorption Isotherms of Limonene Enantiomers on the Surfaces of Cyanuric Acid, Cytosine, Ionol, and Adenine Dinitrate Crystals. Russ. J. Phys. Chem. 94, 2331–2336 (2020). https://doi.org/10.1134/S0036024420110102
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420110102