Abstract
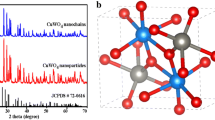
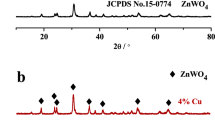
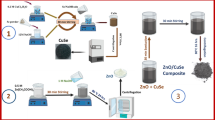
Degradation of organic pollutants got more attention for detoxification of water. In this paper, pure and Zn-doped Cu2O particles were successfully synthesized by water bath co-precipitation method. X-ray diffraction (XRD) study confirmed the cubic structure of Cu2O. Zn doping resulted in reduction in crystallite size without changing d-spacing and crystal structure. Zn doping converts perfect cube to distorted sphere with enhanced surface area that is effective for photocatalytic applications. Elemental study confirmed the uniform distribution of Cu, Zn, and O atoms in the sample. X-ray photoelectron spectra (XPS) analysis showed peak shift in the electronic states of O with higher oxygen vacancy defects. Band gap of Cu2O after Zn doping increased from 1.84 to 1.91 eV. The photocatalytic activity towards methylene blue (MB) dye photodegradation under visible light reached 96% in 120 min after Zn doping compared to 95% in 180 min for pure Cu2O. The improvement in photocatalytic degradation after Zn doping was achieved by the slow electron-hole recombination, band gap increases, oxygen vacancy defects, and higher surface area.








Similar content being viewed by others
REFERENCES
United Nations World Water Assessment Programme, The United Nations World Water Development Report 2017 (UNESCO, Paris, France, 2017).
B. Lin and X. Ouyang, Energy Convers. Manage. 79, 128 (2014).
M. Wainwright, Dyes Pigm. 76, 582 (2008).
V. K. Sharma, Chemosphere 73, 1379 (2008).
M. Rafatullah, O. Sulaiman, R. Hashim, and A. Ahmad, J. Hazard. Mater. 177, 70 (2010).
V. K. Gupta, I. Ali, T. A. Saleh, A. Nayak, and S. Agarwal, RSC Adv. 2, 6380 (2012).
Y. T. Gaim, G. M. Tesfamariam, G. Y. Nigussie, and M. E. Ashebir, J. Compos. Sci. 3, 93 (2019).
S. Álvarez-Torrellas, J. A. Peres, V. Gil-Álvarez, G. Ovejero, and J. García, Chem. Eng. J. 320, 319 (2017).
N. Dorival-García, A. Zafra-Gomez, A. Navalon, J. González, and J. L. Vílchez, Sci. Total Environ. 442, 317 (2013).
Y. Deng and R. Zhao, Curr. Pollut. Rep. 1, 167 (2015).
A. O. Ibhadon and P. Fitzpatrick, Catalysts 3, 189 (2013).
U. I. Gaya, Heterogeneous Photocatalysis Using Inorganic Semiconductor Solids (Springer, Netherlands, 2014), p. 1.
A. Bumajdad and M. Madkour, Phys. Chem. Chem. Phys. 16, 7146 (2014).
X. Wang, F. Wang, Y. Sang, and H. Liu, Adv. Energy Mater. 7, 1700473 (2017).
L. V. Bora and R. K. Mewada, Renewable Sustainable Energy Rev. 76, 1393 (2017).
R. Fagan, D. E. McCormack, D. D. Dionysiou, and S. C. Pillai, Mater. Sci. Semicond. Process. 42, 2 (2016).
N. Srinivasan, M. Anbuchezhiyan, S. Harish, and S. Ponnusamy, Appl. Surf. Sci. 494, 771 (2019).
C. Xu, P. Ravi Anusuyadevi, C. Aymonier, R. Luque, and S. Marre, Chem. Soc. Rev. 48, 3868 (2019).
S. B. A. Hamid, S. J. Teh, and C. W. Lai, Catalysts 7, 93 (2017).
V. Scuderi, G. Amiard, S. Boninelli, S. Scalese, M. Miritello, P. M. Sberna, G. Impellizzeri, and V. Privitera, Mater. Sci. Semicond. Process. 42, 89 (2016).
X. Yu, J. Zhang, J. Zhang, J. Niu, J. Zhao, Y. Wei, and B. Yao, Chem. Eng. J. 374, 316 (2019).
M. Singh, D. Jampaiah, A. E. Kandjani, Y. M. Sabri, E. Della Gaspera, P. Reineck, M. Judd, J. Langley, N. Cox, J. van Embden, E. L. H. Mayes, B. C. Gibson, S. K. Bhargava, R. Ramanathan, and V. Bansal, Nanoscale 10, 6039 (2018).
Y. Jiang, H. Yuan, and H. Chen, Phys. Chem. Chem. Phys. 17, 630 (2015).
L. Zhang, D. Jing, L. Guo, and X. Yao, ACS Sustain. Chem. Eng. 2, 1446 (2014).
D. Sudha and P. Sivakumar, Chem. Eng. Process. Process Intensif. 97, 112 (2015).
N. D. Khiavi, R. Katal, S. K. Eshkalak, S. Masudy-Panah, S. Ramakrishna, and H. Jiangyong, Nanomaterials 9, 1011 (2019).
X. S. Wang, Y. D. Zhang, Q. C. Wang, B. Dong, Y. J. Wang, and W. Feng, Sci. Eng. Compos. Mater. 26, 104 (2019).
L. Liu, W. Yang, W. Sun, Q. Li, and J. K. Shang, ACS Appl. Mater. Interfaces 7, 1465 (2015).
J. X. Kang, T. W. Chen, D. P. Meng, D. F. Zhang, L. Zheng, and L. Guo, Sci. Adv. Mater. 5, 1633 (2013).
F. Hu, Y. Zou, L. Wang, Y. Wen, and Y. Xiong, Int. J. Hydrogen Energy 41, 15172 (2016).
B. Heng, T. Xiao, W. Tao, X. Hu, X. Chen, B. Wang, D. Sun, and Y. Tang, Cryst. Growth. Des. 12, 3998 (2012).
F. Zhang, G. Dong, M. Wang, Y. Zeng, and C. Wang, Appl. Surf. Sci. 444, 559 (2018).
C. P. Goyal, D. Goyal, S. K. Rajan, N. S. Ramgir, Y. Shimura, M. Navaneethan, Y. Hayakawa, C. Muthamizhchelvan, H. Ikeda, and S. Ponnusamy, Crystals 10, 188 (2020).
S. Harish, J. Archana, M. Sabarinathan, M. Navaneethan, K. D. Nisha, S. Ponnusamy, C. Muthamizhchelvan, H. Ikeda, D. K. Aswal, and Y. Hayakawa, Appl. Surf. Sci. 418, 103 (2017).
R. O. Yathisha and Y. Arthoba Nayaka, J. Mater. Sci. 53, 678 (2018).
V. Cretu, V. Postica, A. K. Mishra, M. Hoppe, I. Tiginyanu, Y. K. Mishra, L. Chow, N. H. De Leeuw, R. Adelung, and O. Lupan, J. Mater. Chem. A 4, 6527 (2016).
D. Nath, F. Singh, and R. Das, Mater. Chem. Phys. 239, 122021 (2020).
T. Jiang, Y. Wang, D. Meng, and D. Wang, J. Mater. Sci. Mater. Electron. 27, 12884 (2016).
M. B. Dutt and S. K. Sen, Jpn. J. Appl. Phys. 18, 1025 (1979).
M. Jacquemin, M. J. Genet, E. M. Gaigneaux, and D. P. Debecker, ChemPhysChem 14, 3618 (2013).
L. Zhu, H. Li, Z. Liu, P. Xia, Y. Xie, and D. Xiong, J. Phys. Chem. C 122, 9531 (2018).
R. K. Ratnesh and M. S. Mehata, AIP Adv. 5, 097114 (2015).
Z. C. Ma, L. M. Wang, D. Q. Chu, H. M. Sun, and A. X. Wang, RSC Adv. 5, 8223 (2015).
ACKNOWLEDGMENTS
Authors acknowledge the Center for Instrumental Analysis, Shizuoka University, and Nanotechnology Research Centre, SRM IST for providing characterization facilities.
Funding
We are grateful to SRMIST for providing research facilities and financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. We did not have known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
About this article
Cite this article
Goyal, C.P., Goyal, D., Ganesh, V. et al. Improvement of Photocatalytic Activity by Zn Doping in Cu2O. Phys. Solid State 62, 1796–1802 (2020). https://doi.org/10.1134/S1063783420100091
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063783420100091