Abstract
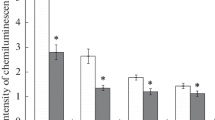
This paper reports that pre-incubation of a neutrophil suspension in the presence of a near-null magnetic field produced using a system of magnetic shields (a residual constant magnetic field not greater than 20 nT) results in a considerable decrease in the intensity of neutrophil lucigenin-dependent chemiluminescence. The addition of the NADPH oxidase inhibitor diphenyliodonium to the incubation medium reduced the chemiluminescence intensity in both the experimental and the control samples (geomagnetic field). It should be noted that the differences observed between the groups, which were caused by the exposure to a near-null magnetic field, are almost the same both at lower (2.5, 5, and 10 μM) and higher (50 and 100 μM) diphenyliodonium concentrations. In contrast, the addition of 2,4-dinitrophenol, an uncoupler of oxidative phosphorylation in mitochondria, in concentrations starting from 5 μM and up to 200 μM almost completely eliminated the difference between the control and experimental samples, which was observed at low inhibitor concentrations, or in its absence.





Similar content being viewed by others
References
H. Zhang, Z. Zhang, W. Mo, et al., Prot. Cell 8 (7), 527 (2017).
C. F. Martino and P. R. Castello, PLoS One 6 (8), e22753 (2011).
P. Politanski, E. Rajkowska, M. Brodecki, et al., Bioelectromagnetics 34, 333 (2013).
V. N. Binhi and F. S. Prato, PLoS One 12 (6), e0179340 (2017).
V. V. Novikov, E. V. Yablokova, and E. E. Fesenko, Biophysics (Moscow) 63 (3), 365 (2018).
V. V. Novikov, E. V. Yablokova, E. R. Valeeva, and E. E. Fesenko, Biophysics (Moscow) 64 (4), 571 (2019).
V. V. Novikov, E. V. Yablokova, I. A. Shaev, and E. E. Fesenko Biophysics (Moscow) 65 (3), 524 (2020).
J. P. Crow, Nitric Oxide Biol. Chem. 1 (2), 145 (1997).
S. L. Hempel, G. R. Buettner, Y. Q. O’Malley, et al., Free Radic. Biol. Med. 27 (1–2), 146 (1999).
G. Bartosz, Clin. Chim. Acta 368, 53 (2006).
T. B. Aasen, B. Bolann, J. Glette, et al., Scand. J. Clin. Lab. Invest. 47, 673 (1987).
A. A. Dzhatdoeva, E. V. Proskurnina, A. M. Nesterova, et al., Biol. Membrany 34 (6), 116 (2017).
A. R. Cross and O. T. Jones, Biochem. J. 237, 111 (1986).
Y. Li and M. A. Trush, Biochim. Biophys. Acta 253, 295 (1998).
S. Matsuyama, J. L. Lopis, Q. L. Deveraux, et al., Nat. Cell. Biol. 2, 318 (2000).
M. M. El-Guindy, A. C. Neder, and C. B. Gomes, Cell Mol Biol. 27 (5), 399–402 (1981).
V. V. Novikov, E. V. Yablokova, and E. E. Fesenko, Biophysics (Moscow) 65 (1), 82 (2020).
V. V. Novikov, I. M. Sheiman, and E. E. Fesenko, Biophysics (Moscow) 52 (5), 498 (2007).
V. V. Novikov, I. M. Sheiman, and E. E. Fesenko, Bioelectromagnetics 29, 387 (2008).
S. W. Edwards, Biochemistry and Physiology of the Neutrophil (Cambridge Univ. Press, New York, 1994).
M. L. Karnovsky, Semin. Hematol. 5, 156 (1968).
S. W. Edwards, M. B. Hallett, and A. K. Campbell, Biochem. J. 217, 851 (1984).
A. W. Segal and A. Abo, Trends Biochem. Sci. 18, 43 (1993).
J. G. Pryde, A. Walker, A. G. Rossi, et al., J. Biol. Chem. 275, 33574 (2000).
G. Fossati, D. A. Moulding, D. G. Spiller, et al., J. Immunol. 170, 1964 (2003).
A. Panday, M. K. Sahoo, D. Osorio, and S. Batra, Cell Mol. Immunol. 12, 5 (2015).
V. Kozjak-Pavlovic, Cell Tissue Res. 367 (1), 83 (2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest. Authors declare no conflict of interest.
Additional information
Translated by E. Martynova
Abbreviations: ROS, reactive oxygen species; MF, magnetic field; SAR, superoxide anion radical.
Rights and permissions
About this article
Cite this article
Novikov, V.V., Yablokova, E.V., Shaev, I.A. et al. Decreased Production of the Superoxide Anion Radical in Neutrophils Exposed to a Near-Null Magnetic Field. BIOPHYSICS 65, 625–630 (2020). https://doi.org/10.1134/S0006350920040120
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350920040120