Abstract
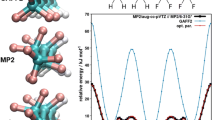
The possibility for tetra-, hexa-, octa-, and decanitrogen to exist was analyzed using the QCISD(T)/TZVP and G4 quantum chemical calculations. The results obtained suggest the existence of only four allotropes of nitrogen whose molecules contain from four to eight atoms, viz., rectangular and tetrahedral N4, open-book N6, and cubic N8. The bond lengths and bond angles were calculated for all compounds, as well as selected thermodynamic parameters (standard enthalpy of formation ΔfHo, standard entropy of formation So, standard Gibbs energy of formation ΔfGo) of the compounds in the gas phase. The enthalpies and entropies of the oxidation reactions of each compound by molecular oxygen were calculated using the results of quantum chemical computations. All the oxidation reactions are highly exothermic and practically irreversible; therefore, the title allotropes of nitrogen may appear to be promising combustible materials.
Similar content being viewed by others
References
Khimicheskaya entsiklopediya [The Chemical Encyclopedia], Vol. 1, Sov. Entsiklopediya, Moscow, 1988, p. 58 (in Russian).
F. Cacace, G. de Petris, A. Troiani, Science, 2002, 295, 480.
M. M. Francl, J. P. Chesick, J. Phys. Chem., 1990, 94, 526.
A. A. Bliznyuk, M. Shen, H. F. Schaefer III, Chem. Phys. Lett., 1992, 198, 249.
T.-K. Ha, O. Suleimenov, M. T. Nguyen, Chem. Phys. Lett., 1999, 315, 327.
S. L. Qian, L. J. Wang, W. G. Xu, Theor. Chem. Acc., 2000, 104, 67.
H.-X. Duan, S. L. Qian, Chem. Phys. Lett., 2006, 432, 331.
W. J. Lauderdale, J. F. Stanton, R. J. Bartlett, J. Phys. Chem., 1992, 96, 1173.
M. Tobita, R. J. Bartlett, J. Phys. Chem. A, 2001, 105, 4107.
M. T. Nguyen, Coord. Chem. Rev., 2003, 244, 93.
A. Smirnov, D. Lempert, T. Pivina, D. Khakimov, Centr. Eur. J. Energ. Mater., 2011, 8, 233.
V. F. Elesin, N. N. Degtyarenko, K. S. Pazhitnykh, N. V. Matveev, Russ. Phys. J., 2009, 52, 1224.
X. Wang, F. Tian, L. Wang, T. Cui, B. Liu, J. Chem. Phys., 2010, 132, 024502.
L. Türker, Defence Technol., 2018, 14, 19.
L. Türker, Defence Technol., 2019, 15, 154.
L. J. Wang, P. G. Mezey, M. Z. Zgierski, Chem. Phys. Lett., 2004, 391, 338.
B. M. Gimarc, M. Zhao, Inorg. Chem., 1996, 35, 3289.
T. M. Klapötke, R. D. Harcourt, J. Mol. Struct. (Theochem), 2001, 541, 237.
M. Noyman, S. Zilberg, Y. Haas, J. Phys. Chem. A, 2009, 113, 7376.
P. C. Samartzis, A. M. Woodtke, Inter. Rev. Phys. Chem., 2006, 25, 1952.
E. G. Lewars, Modeling Marvels: Computational Anticipation of Novel Molecules, Springer Science+Business Media, 2008, p. 141.
G. Zhou, J.-L. Zhang, N.-B. Wong, J. Mol. Struct. (Theochem), 2004, 668, 189.
J. A. Pople, M. Head-Gordon, K. Raghavachari, J. Chem. Phys., 1987, 87, 5968.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, D. J. Fox, Gaussian 09, Revision A.01, Gaussian, Inc., Wallingford CT, 2009.
L. A. Curtiss, P. C. Redfern, K. Raghavachari, J. Chem. Phys., 2007, 126, 084108.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 11, pp. 2067—2072, November, 2020.
Rights and permissions
About this article
Cite this article
Chachkov, D.V., Mikhailov, O.V. Tetra-, hexa-, and octanitrogen molecules: a quantum chemical design and thermodynamic properties. Russ Chem Bull 69, 2067–2072 (2020). https://doi.org/10.1007/s11172-020-3001-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-020-3001-6