Abstract
Heterozygous mutations in the ACAN gene have been reported in individuals with short stature and advanced bone age, with or without early-onset osteoarthritis and/or osteochondritis dissecans. We report a family with a phenotypic constellation carrying a novel mutation in the ACAN gene. The proband was a 7-year-old Japanese girl with short stature. Her mother and maternal grandmother also had short stature and intervertebral disc disease. We analyzed the ACAN gene in the family and identified a novel heterozygous mutation: c.4634delT, Leu1545Profs*11.
Similar content being viewed by others
Aggrecan, encoded by the ACAN gene, is a cartilage-specific proteoglycan core protein that is a major structural component of the cartilage growth plate and intervertebral disc. Heterozygous ACAN mutations have been reported in individuals with short stature and advanced bone age, with or without early-onset osteoarthritis and/or osteochondritis dissecans (SSOAOD) (OMIM # 165800)1,2,3. The mutations identified to date are located throughout the protein, and more than half of them are premature truncating mutations1,2,3. Overall, the average adult height between individuals with truncating and missense mutations does not differ1. Except for an association with osteochondritis dissecans for two missense mutations located at the C-terminal C-type lectin domain, no definite genotype–phenotype correlation has been found1,2. The ACAN gene has a variable number tandem repeat (VNTR) at exon 12, which encodes the chondroitin sulfate attachment domain that dictates the length of the aggrecan core protein. We report a family with SSOAOD caused by a novel frameshift mutation at 234 bp from the 3′ end of the VNTR in ACAN.
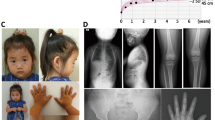
The proband was a Japanese girl who is the third child of nonconsanguineous parents. She was delivered at 37 weeks of gestation with a birth length of 43.3 cm (−0.1 SD) and weight of 2350 g (−1.1 SD). Short stature had manifested since she was 1 year and 6-months old (Fig. 1A). She underwent endocrine assessment at 3 years of age, yielding normal results. She was referred to our hospital for further examinations at the age of 7 years and 8 months. Her height was 105.1 cm (−3.6 SD), weight was 19.4 kg (−1.4 SD), and arm span was 103 cm. She was noted to have a prominent jaw as a result of midface hypoplasia, a short left 4th toe, and broadening of the bilateral 5th toes. A skeletal survey showed no characteristic signs of skeletal dysplasia, other than minor changes of the digits, including mild shortening of the 4th and 5th metacarpals, shortening of the 1st distal phalanx (Fig. 1B), cone-shaped epiphyses of the left 1st and 4th metatarsals, and large epiphyses of the 5th distal phalanges (Fig. 1C). According to the Greulich and Pyle method, her bone age was 8 years and 10 months (Fig. 1B). The proband’s mother was 136.8 cm (−4.2 SD) tall, and her skeletal X-ray showed narrowed intervertebral spaces and endplate irregularities of the lower thoracic and upper lumbar vertebrae, indicative of early-onset intervertebral disc disease (Fig. 1D). The maternal grandmother was 134.4 cm (−4.7 SD) tall. She also had severe intervertebral disc disease and had undergone surgery for lumbar disc herniation (Fig. 1E). Other members of the family had an average height (Fig. 1F).
A Growth chart of the proband. Heights and weights are shown as black points. B X-ray of the proband’s left hand. Mild shortening of the 4th and 5th metacarpals and shortening of the 1st distal phalanx are shown. The bone age was interpreted as 8 years and 10 months using the Greulich and Pyle method. C X-ray of the proband’s feet. Cone-shaped epiphyses of the left 1st and 4th metatarsals and large epiphyses of the 5th distal phalanges are shown. D, E Lumbar spine X-rays of the proband’s mother and maternal grandmother. Narrowed intervertebral spaces and endplate irregularities are shown. F Pedigree of the studied family. The arrow indicates the proband (III-3). The family members with short stature carrying ACAN mutations are indicated with filled symbols. Ages and heights in standard division score are reported below each symbol.
After genetic counseling, we obtained informed consent from the family. The study was approved by the Ethics Committee of the Keio University School of Medicine. We extracted genomic DNA from peripheral blood samples of affected members of the family and amplified ACAN (NM_013227.3) coding exons and flanking introns, except for the regions flanking the VNTR in exon 12, as described previously4,5,6. To cover the regions flanking the VNTR, we designed additional sets of primers and performed polymerase chain reaction (details are provided in Supplementary tables). We also performed direct sequencing of the amplified products. DNA fragment sizes of the VNTR indicated 27 repeats in both the proband and her mother and 28 in the grandmother, which are common repeat numbers found in healthy adults7,8. By sequencing each side of the VNTR, we identified a novel heterozygous variant (c.4634delT, p.(Leu1545Profs*11)) at 234 bp from the 3′ end of the VNTR in the proband, mother, and maternal grandmother (Fig. 2). Genetic testing of other members was not permitted. This variant was not found in the Human Genetic Variation Database (HGVD; http://www.hgvd.genome.med.kyoto-u.ac.jp) or Genome Aggregation Database (gnomAD; https://gnomad.broadinstitute.org/). We conclude that this variant is a novel pathogenic mutation predicted to cause premature termination of translation and nonsense-mediated mRNA decay, leading to haploinsufficiency of ACAN.
A The black area on the exon 12 denotes various numbers tandem repeat. Variant c.4634delT (black triangle) is reported in this study. B Partial sequence (reverse complemented). The upper panel shows a chromatogram of the proband that had a heterozygous mutation, c.4634delT, Leu1545Profs*11, which is denoted by an arrow. The lower panel shows a chromatogram of the wild-type sequence.
The diagnosis of SSOAOD in this family is convincing. Clinically, some points are noteworthy. First, the short stature showed an autosomal dominant inheritance. Second, the proband had advanced bone ages of 1 year and 2 months compared to her chronological age, though not all previous patients with SSOAOD showed advanced bone age1. Third, the mother and grandmother had intervertebral disc diseases, consistent with a previous report by Dateki indicating that several cases of SSOAOD have an early onset and multiple intervertebral disc diseases3. Fourth, the proband had midface hypoplasia, a short left 4th toe and broad 5th toes. There have been reports on individuals with SSOAOD exhibiting midface hypoplasia and shortening or broadening of digits1,2,3. The phenotype of the proband suggested that mild dysmorphic findings may constitute syndromic components of ACAN mutations. SSOAOD should be considered in the differential diagnosis of children with autosomal dominant short stature, particularly in cases in which there is advanced bone age or a family history of intervertebral disc disease.
In conclusion, we identified a novel heterozygous ACAN mutation. Our report provides further evidence that ACAN mutations are associated with autosomal dominant short stature with intervertebral disc disease.
HGV database
The relevant data from this Data Report are hosted at the Human Genome Variation Database at https://doi.org/10.6084/m9.figshare.hgv.2948.
References
Gkourogianni, A. et al. Clinical characterization of patients with autosomal dominant short stature due to aggrecan mutations. J. Clin. Endocrinol. Metab. 102, 460–469 (2017).
Dateki, S. ACAN mutations as a cause of familial short stature. Clin. Pediatr. Endocrinol. 26, 119–125 (2017).
Sentchordi-Montané, L. et al. Heterozygous aggrecan variants are associated with short stature and brachydactyly: description of 16 probands and a review of the literature. Clin. Endocrinol. 88, 820–829 (2018).
Doege, K. J., Coulter, S. N., Meek, L. M., Maslen, K. & Wood, J. G. A human-specific polymorphism in the coding region of the aggrecan gene: variable number of tandem repeats produce a range of core protein sizes in the general population. J. Biol. Chem. 272, 13974–13979 (1997).
Stattin, E. L. et al. A missense mutation in the aggrecan C-type lectin domain disrupts extracellular matrix interactions and causes dominant familial osteochondritis dissecans. Am. J. Hum. Genet. 86, 126–137 (2010).
Quintos, J. B., Guo, M. H. & Dauber, A. Idiopathic short stature due to novel heterozygous mutation of the aggrecan gene. J. Pediatr. Endocrinol. Metab. 28, 927–932 (2015).
Mashayekhi, F., Shafiee, G., Kazemi, M. & Dolati, P. Lumbar disk degeneration disease and aggrecan gene polymorphism in Northern Iran. Biochem. Genet. 48, 684–689 (2010).
Solovieva, S. et al. Association between the aggrecan gene variable number of tandem repeats polymorphism and intervertebral disc degeneration. Spine 32, 1700–1705 (2007).
Acknowledgements
This study was partly supported by the Japan Agency for Medical Research and Development (AMED) (17bm0804012 h0001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T.H. has the following financial relationships to disclose: research funding from Novo Nordisk Pharma Ltd. and JCR Pharmaceuticals Co., Ltd.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Uchida, N., Shibata, H., Nishimura, G. et al. A novel mutation in the ACAN gene in a family with autosomal dominant short stature and intervertebral disc disease. Hum Genome Var 7, 44 (2020). https://doi.org/10.1038/s41439-020-00132-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41439-020-00132-8
This article is cited by
-
Diagnostic utility of next-generation sequencing-based panel testing in 543 patients with suspected skeletal dysplasia
Orphanet Journal of Rare Diseases (2021)