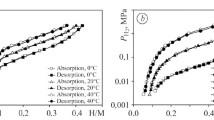
The free energy and density of states are computed by the DFT method for the Zr3NiO and Zr3NiN compounds. Their formation was confirmed and numerous Zr3MNx (M = Co, Ni) compounds were synthesized for the first time. It is shown that all these compounds are insertion derivatives of the Re3B structural type (space group Cmcm). We study the process of gas-phase hydrogenation of the obtained subnitrides. It is shown that some specimens form single-phase hydrides (Zr3NiN0.5H5.64 and Zr3CoNH5.62) preserving the crystal structure of the original matrix with an increase in the volume of elementary cell by ∼ 16%. The process of hydrogen desorption in a vacuum was studied by the TDS method for various Zr3CoN(O)x hydrides. We compare the desorption properties of hydrides depending on the type of stabilizing element and the hydrogen content of hydride.




Similar content being viewed by others
References
B. Rupp, “On the change in physical properties of Ti4–xFe2+xOy during hydrogenation I: Activation behavior of ternary oxides Ti4–x Fe2+xOy and β-Ti,” J. Less-Common Met., 104, 51–63 (1984).
M. H. Mintz, Z. Hadari, and M. P. Dariel, “Hydrogenation characteristics of Ti2NiOx compounds (0 ≤ x < 0.5),” J. Less-Common Met., 63, 181–191 (1979)
H. Boller, “Über den aufgefüllten Re3B-Typ in den Systemen (Zr, Hf)–(Fe, Co, Ni)–O,” Monatsh. Chem., 104, 545–549 (1973).
P. Rogl, H. Nowotny, and F. Benesowsky, “Neue K-Boride und verwandte Phasen (Re3B-Typ, aufgefüllt),” Monatsh. Chem., 104, 182–193 (1973).
I. Zavaliy, A. Riabov, V. Yartys, G. Wiesinger, H. Michor, and G. Hilscher, “(Hf, Zr)2 Fe and Zr4Fe2O0.6 compounds and their hydrides: phase equilibria, crystal structure, and magnetic properties,” J. Alloys Comp., 265, 6–14 (1998).
I. Yu. Zavaliy, R. V. Denys, I. V. Koval’chuck, A. В. Riabov, and R. G. Delaplane, “Hydrogenation of Ti4–xZrxFe2Oy alloys and crystal structure analysis of their deuterides,” Chem. Met. Alloys, 2, 59–67 (2009).
I. Zavaliy, “Effect of oxygen content on hydrogen storage capacity of Zr-based η-phases,” J. Alloys Comp., 291, 102–109 (1999).
I. Yu. Zavaliy, W. B. Yelon, P. Yu. Zavalij, I. V. Saldan, and V. K. Pecharsky, “The crystal structure of the oxygen-stabilized η-phase Zr3V3OxD9.6,” J. Alloys Comp., 309, 75–82 (2000).
I. Y. Zavalii, R. Cerny, I. V. Koval’chuck, and I. V. Saldan, “Hydrogenation of oxygen-stabilized Zr3NiOx compounds,” J. Alloys Comp., 360, 173–182 (2003).
I. Yu. Zavaliy, R. V. Denys, R. Cerný, I. V. Koval’chuck, G. Wiesinger, and G. Hilscher, “Hydrogen-induced changes in crystal structure and magnetic properties of the Zr3MOx (M = Fe, Co) phases,” J. Alloys Comp., 386, 26–34 (2005).
I. V. Koval’chuck, R. Cerny, R. V. Denys, and I. Yu. Zavaliy, “Crystal structure of k-Hf9Mo4SiD16.8 deuteride,” Chem. Met. Alloys, 1, 180–184 (2008).
I. Zavaliy, G. Wojcik, G. Mlynarek, I. Saldan, V. Yartys, and M. Kopczyk, “Phase-structural characteristics of (Ti1−xZrx)4Ni2O0.3 alloys and their hydrogen gas and electrochemical absorption-desorption properties,” J. Alloys Comp., 314, 124–131 (2001).
I. V. Saldan, Yu. H. Dubov, O. B. Ryabov, and I. Yu. Zavaliy, “Effect of the modification of metal-hydride electrodes based on Ti2Ni alloys on their discharge characteristics,” Fiz.-Khim. Mekh. Mater., 42, No. 5, 56–64 (2006); English translation: Mater. Sci., 42, No. 5, 634–643 (2006).
I. Yu. Zavalii, Yu. V. Verbovyts’kyi, V. V. Berezovets’, V. V. Shtender, V. K. Pecharsky, and P. Ya. Lyutyi, “Synthesis, structure, and hydrogen-sorption properties of (Ti, Zr)4Ni2Nx subnitrides,” Fiz.-Khim. Mekh. Mater., 53, No. 3, 18–25 (2017); English translation: Mater. Sci., 53, No. 3, 306–315 (2017).
L. G. Akselrud, Yu. M. Grin, P. Yu. Zavalij, and V. K. Pecharsky, “Use of the CSD program package for structure determination,” in: Proc. 2nd Europ. Powder Diffraction Conf., Enschede, Netherlands, R. Delhez and E. J. Mittemijer (editors) (1993), p. 41.
R. Sundararaman, K. Letchworth-Weaver, K. A. Schwarz, D. Gunceler, Y. Ozhabes, and T. A. Arias, “JDFTx: Software for joint density-functional theory,” SoftwareX, 6, 278–284 (2017).
C. Freysoldt, S. Boeck, and J. Neugebauer, “Direct minimization technique for metals in density functional theory,” Phys. Rev. B, 79, I. 241103(R) (2009).
M. Schlipf and F. Gygi, “Optimization algorithm for the generation of ONCV pseudopotentials,” Comput. Phys. Comm., 196, 36–44 (2015).
J. P. Perdew, K. Burkeand, and M. Ernzerhof, “Generalized gradient approximation made simple,” Phys. Rev. Lett., 77, 3865–3868 (1996).
S. L. Dudarev, G. A. Botton, S. Y. Savrasov, C. J. Humphreys, and A. P. Sutton, “Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study,” Phys. Rev. B, 57, 1505–1509 (1998).
J. W. Bennett, B. G. Hudson, I. K. Metz, D. Liang, S. Spurgeon, Q. Cui, and S. E. Mason, “A systematic determination of Hubbard U using the GBRV ultrasoft pseudopotential set,” Comput. Mater. Sci., 170, I. 109137 (2019).
A. Chikdene, A. Baudry, J. L. Soubeyroux, P. Boyer, S. Miraglia, and D. Fruchart, “Neutron diffraction studies of Zr2NiH(D)x hydrides,” Z. Phys. Chem., 163, 219–224 (1989).
R. W. G. Wyckoff, Crystal Structures, Interscience, Vol. 1, New York (1963), pp. 7–83.
J. E. Jörgensen and R. I. Smith, “On the compression mechanism of FeF3,” Acta Crystallogr. B, 62, 987–992 (2006).
A. Dubertret and P. Lehr, “Description d’unesur structure Zr3O1–x,” C. R. Math, Acad. Sci. Paris, 267, 820–822 (1968).
A. B. Riabov, V. A. Yartys, G. Wiesinger, B. C. Hauback, P. W. Guegan, and I. R. Harris, “Hydrogenation behavior, neutron diffraction studies, and microstructural characterization of boronoxide-doped Zr–V alloys,” J. Alloys Comp., 293, 93–100 (1999).
H. Fukui, M. Fujimoto, Y. Akahama, A. Sano-Furukawa, and T. Hattori, “Structure change of monoclinic ZrO2 baddeleyite involving softenings of bulk modulus and atom vibrations,” Acta Crystallogr. B, 75, 742–749 (2019).
J. Gatterer, G. Dufek, P. Ettmayer, and P. Kieffer, “Daskubische Tantalmononitrid (B1-Typ) und seine Mischbarkeitmit den isotypen Übergangs metall-nitriden und carbiden,” Monat. Chem. Verw., 106, 1137–1147 (1975).
L. Thomassen, “An X-ray investigation of the system Cr2O3–NiO,” J. Amer. Chem. Soc., 62, 1134–1135 (1940).
A. Leineweber, H. Jacobs, and S. Hull, “Ordering of nitrogen in nickel nitride Ni3N determined by neutron diffraction,” Inorg. Chem., 40, 5818–5822 (2001).
A. B. Riabov, V. A. Yartys, H. Fjellvåg, B. C. Hauback, and M. H. Sørby, “Neutron diffraction studies of Zr-containing intermetallic hydrides with ordered hydrogen sublattice: V. Orthorhombic Zr3CoD6.9 with filled Re3B-type structure,” J. Alloys Comp., 296 (1–2), 312–316 (2000).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 56, No. 3, pp. 93–102, May–June, 2020
Rights and permissions
About this article
Cite this article
Zavalii, I.Y., Lyutyi, P.Y., Oshchapovskyi, I.V. et al. New Zr3MNx (M = Co, Ni) Subnitrides: Theoretical Analyses, Crystal Structure, and Hydrogen-Sorption Properties. Mater Sci 56, 389–399 (2020). https://doi.org/10.1007/s11003-020-00442-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-020-00442-w