Abstract
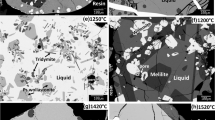
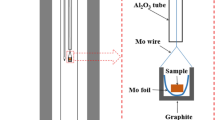
X-ray powder diffraction and Rietveld full-profile analysis are used to establish the homogeneity ranges and crystalline structure of solid solutions Nd2−zCazO3−z/2 (0.0 ≤ z ≤ 0.1, space group P\(\bar {3}\)m1) and Nd2−yCayCoO4−δ (0.7 ≤ y ≤ 1.0, space group I 4/mmm). Dependences of the unit cell parameters on the composition of solid solution Nd2−yCayCoO4−δ are obtained. It is shown that all cobaltites Nd2−yCayCoO4−δ in the temperature range of 298–1373 K in air possess virtually stoichiometric composition. Average values of thermal expansion coefficients for Nd2−yCayCoO4−δ (y = 0.8; 0.9) are calculated. An isobaric-isothermal section of the phase diagram for the 1/2Nd2O3–CaO–CoO system at 1373 K in the air has been constructed.





Similar content being viewed by others
REFERENCES
A. N. Petrov, V. A. Cherepanov, A. Yu. Zuyev, et al., J. Solid State Chem. 77, 1 (1988).
H. Hashimoto, T. Kusunose, and T. Sekino, J. Alloys Compd. 484, 246 (2009).
C. Tealdi, M. S. Islam, C. A. J. Fisher, et al., Prog. Solid State Chem. 35, 491 (2007).
V. R. Choudhary and K. C. Mondal, Appl. Energy 83, 1024 (2006).
L. Malavasi, C. Teadi, G. Flor, et al., Sens. Actuators 105, 407 (2005).
A. Patil, S. C. Parida, S. Dash, et al., Thermochim. Acta 465, 25 (2007).
Z. Ali, I. Ahmad, B. Amin, et al., Phys. B (Amsterdam, Neth.) 406, 3800 (2011).
M. Bose, A. Ghoshray, and A. Basu, Phys. Rev. B 26, 4871 (1982).
M. V. Kniga, I. I. Vygovskii, and E. E. Klimentovich, Zh. Neorg. Khim. 24, 1171 (1979).
K. Kitayama, J. Solid State Chem. 137, 255 (1998).
K. Kitayama, J. Solid State Chem. 76, 241 (1988).
A. Olafser, H. Fjellvag, and B. C. Hauback, J. Solid State Chem. 151, 46 (2000).
A. N. Petrov, V. A. Cherepanov, and A. Yu. Zuev, Zh. Fiz. Khim. 61, 630 (1987).
H. Tran, T. Mehta, M. Zeller, et al., Mater. Res. Bull. 48, 2450 (2013).
K. Vidyasagar, J. Gopalakrishnan, and C. N. R. Rao, Inorg. Chem. 23, 1206 (1984).
K. Agilandeswari and A. Ruban Kumar, Adv. Powder Technol 25, 904 (2014).
J. Pei, G. Chen, X. Li, et al., Mater. Lett. 63, 1459 (2009).
A. Sotelo, Sh. Rasekh, M. A. Torres, et al., J. Solid State Chem. 221, 247 (2015).
C. H. Hervoches, H. Fjellva, A. Kjekshus, et al., J. Solid State Chem. 180, 628 (2007).
K. Iwasaki, H. Yamane, S. Kubota, et al., J. Alloys Compd. 358, 210 (2003).
E. Woermann and A. Muan, J. Inorg. Nucl. Chem. 32, 1455 (1970).
K. T. Jacob and P. Gupta, J. Solid State Chem. 221, 57 (2015).
W. Wong-Ng, W. Laws, K. R. Talley, et al., J. Solid State Chem. 215, 128 (2014).
H. Taguchi, H. Kido, and K. Tabata, Phys. B (Amsterdam, Neth.) 344, 271 (2004).
O. A. Shlyakhtin, G. N. Mazo, M. S. Kaluzhskikh, et al., Mater. Lett. 75, 20 (2012).
O. A. Shlyakhtin, G. N. Mazo, S. A. Malyshev, et al., Mater. Res. Bull. 48, 245 (2013).
G. J. Thorogood, P. Orain, M. Ouvry, et al., Solid State Sci. 13, 22113 (2011).
T. V. Aksenova, Sh. I. Elkalashy, A. S. Urusova, and V. A. Cherepanov, Russ. J. Inorg. Chem. 62, 1090 (2017).
A. S. Urusova, V. A. Cherepanov, T. V. Aksenova, et al., J. Solid State Chem. 202, 207 (2013).
A. P. Galayda, N. E. Volkova, L. Ya. Gavrilova, et al., J. Alloys Compd. 718, 288 (2017).
W. Wong-Ng, W. Laws, S. H. Lapidus, et al., Solid State Sci. 48, 31 (2015).
R. D. Shannon, Acta Crystallogr. 32, 751 (1976).
X. Liu and C. T. Prewitt, J. Phys. Chem. Solids 52, 441 (1991).
A. R. Gilev, E. A. Kiselev, and V. A. Cherepanov, RSC Adv. 6, 72905 (2016).
A. R. Gilev, E. A. Kiselev, D. M. Zakharov, et al., Solid State Sci. 72, 134 (2017).
A. R. Gilev, E. A. Kiselev, D. M. Zakharov, et al., J. Alloys Compd. 753, 491 (2018).
ACKNOWLEDGMENTS
This work was supported by the RF Ministry of Science and Higher Education, project no. FEUZ-2020-0052; and by RF Government Program 211, contract no. 02.A03.21.0006.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Drozdova
Rights and permissions
About this article
Cite this article
Aksenova, T.V., Urusova, A.S. & Cherepanov, V.A. Phase Equilibria and Structure of Complex Oxides in the 1/2 Nd2O3–CaO–CoO System in Air at 1373 K. Russ. J. Phys. Chem. 94, 2495–2501 (2020). https://doi.org/10.1134/S0036024420120031
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420120031