Abstract
Geographical limits of species’ distributions are assumed to be coincident with ecological margins, although this assumption might not always be true. Indeed, harsh environments such as Alpine and Mediterranean ecosystems may favour high phenotypic variability among populations, especially those in peripheral sites. Floral traits are often found to be less variable and less affected by environmental heterogeneity than vegetative traits because variation in the former may have negative effects on fitness. For this reason, it is important to quantify variation in floral traits and plant fecundity in study range limits. The objective of the study is to examine phenotypic variation and differences in reproduction in endemic Lilium pomponium in the Maritime and Ligurian Alps in relation to environmental variation across its distribution range. In this species, marginal climatic populations occur both in the peripheral and central geographical locations of the distribution range; hence, geographical and ecological gradients are not concordant. Floral trait variation is related to local environmental conditions with an array of interactions among resource availability, potential pollen limitation and population size that are differentially related to floral traits. Contrary to the general expectation, all central and peripheral populations had similar, moderate seed production with each group limited by different factors acting on different stages of the life-history strategy. Our results are in line with the idea that general expectations are confirmed only when its assumptions are met and that the differences in pollination environment along an environmental gradient may not be the main determinant of the distribution limit.
Similar content being viewed by others
Introduction
There is much interest in ecology and evolution in the occurrence of trait variation from the centre towards the geographical periphery of species’ distributions (Sagarin and Gaines 2002; Eckert et al. 2008; Pironon et al. 2015). In particular, as the centre–periphery hypothesis (CPH) predicts, geographically isolated peripheral populations are expected to be divergent from central populations and to be smaller, less abundant and more isolated from each other than central populations, features that are likely to significantly affect levels of both neutral and adaptive genetic diversity when compared to central populations (Pironon et al. 2017). In plants, a decline in habitat quality towards the periphery of the range is expected to cause either a decline in population size and density resulting in reduced pollinator service (Stone and Jenkins 2008; Moeller et al. 2012) or inadequate pollination driving the decline of population size (Hegland and Totland 2005; Moeller et al. 2012) and, whatever the case, little potential for further range expansion. These responses assume that environmentally marginal populations occur at the geographical periphery of species range with inadequate pollination at the range margins. The loss of stigma-height polymorphism in peripheral populations of different Narcissus species in the Mediterranean region fit this schema (Barrett et al. 2004; Papuga et al. 2015).
However, the assumption of concordance between geographical periphery and environmental marginality has received increasing criticism (Soulé 1973; Pironon et al. 2017). First, peripheral populations may occur in conditions similar to those in the centre of the range (Piñeiro et al. 2007; Kropf et al. 2008). Second, environmental factors may impose ecologically marginal conditions in any part of the species’ range (Soulé 1973—hereafter “marginal populations”). Third, geographically peripheral populations may not occur in marginal conditions but simply in different ecological conditions (Papuga et al. 2018). In particular, in Alpine and Mediterranean ecosystems, environmental factors change over very short distances because of the high topographic complexity (e.g. Körner 2003; Thompson 2001; Doxa and Prastacos 2020), leading to differences in abiotic and biotic resources not necessarily associated with different parts of the range. Such highly heterogeneous and mosaic environments may favour high phenotypic variability (Graae et al. 2018). For all these reasons, geographically peripheral populations are of fundamental importance to studies of species’ range limits (Lesica and Allendorf 1995; Hampe and Petit 2005).
In general, floral traits are found to be less variable and less affected by environmental heterogeneity than vegetative traits because variation in floral morphology may have negative effects on fitness (Berg 1960; Frazee and Marquis 1994). Nevertheless, population variation has been detected in stigma–anther separation (Griffin and Willi, 2014; Papuga et al. 2015), floral display (Dai et al. 2017; Lambrecht et al. 2017), and pollen–ovule ratio (Guo et al. 2010; Dai et al. 2017). This variation in floral traits is usually related to variation in the pollination environment (Aigner 2004) and some studies have detected increased pollen limitation at the distributional edges (Moeller et al 2012), although others do not record any increase in pollen limitation, presumably due to increase in resource constraints or self-pollination (Totland 2001; Hargreaves et al 2015). Assessing the relationship between environmental variation and traits related to pollination environment may increase our understanding of factors shaping distribution limits and species persistence. However, relatively few studies have been conducted to understand the relationship between floral trait variation and environmental variation across the range (but see Gamble et al 2018; Seguí et al 2018).
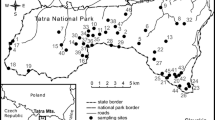
Lilium pomponium L. is a self-incompatible perennial geophyte endemic to the Maritime and Ligurian Alps (Fig. 1) that grows on calcareous outcrops from 100 to 2000 m altitude, from a typical Mediterranean climate to a cool-summer continental type climate in subalpine habitats (Casazza et al 2018). This species thus provides an excellent model for studying traits related to pollination across an environmental gradient. The objective of the present study is to examine whether phenotypic and reproductive traits are related to environmental and geographical gradient across the distribution range of L. pomponium L. Because of the rough topography of the study area, we explicitly took into account climatic conditions rather than using altitude as their proxy in assessing environmental marginality. Specifically, the goals of this study were (1) to assess whether geographically peripheral populations are also environmentally marginal; (2) to assess whether these environmentally marginal populations differ phenotypically from central populations in floral traits, and whether they show differences in reproductive output and mating system.
Map showing the geographic location of the 20 populations of Lilium pomponium used in this study. Symbols indicate the four climatic groups: coastal marginal (hexane ring), central populations (unfilled up-pointing triangle); inland marginal (unfilled rectangle), and subalpine marginal (unfilled Lozenge). Filled circle = geographic centre (GC). Populations’ codes are those reported in Table 1
Materials and methods
Study species
Lilium pomponium L. (Liliaceae) has hermaphrodite flowers although male flowers can occur (ca. 10%, personal observation). Anthesis usually lasts from May to July and capsules develop from late July to September, according to local climatic conditions. Reproductive output is low, flowers are self-incompatible (Casazza et al. 2018) and show ‘approach herkogamy’, that is, the stigmas are above the anther levels (Fryxell 1957; Webb and Lloyd 1986). These traits limit self-pollination and promote outcrossing (Webb and Lloyd 1986) and reduce sexual interference (Barrett 2002). Seeds are very thin with a winged margin for wind dispersal (pterometeorochory) that enables herbs to reach roughly 15 m (Vittoz and Engler 2007).
Study occurrences and climatic data
The distributional range of L. pomponium extends from the Neva Valley in northwest Italy to the Verdon Valley in southwest France (Fig. 1). Species occurrences were obtained from field surveys (performed by the authors) and from regional databases: SILENE (Conservatoire botanique national méditerranéen de Porquerolles; http://flore.silene.eu/) and LiBiOss (Regione Liguria; http://www.cartografiarl.regione.liguria.it/Biodiv/Biodiv.aspx). Occurrences were spatially filtered and those closer than 1 km to each other were removed, resulting in a final data set of 809 occurrences. To study climatic conditions of populations, we downloaded nineteen bioclimatic variables representative of the period 1979–2013 from the CHELSA climate database website (http://chelsa-climate.org/) at 30-s (c. 1 km) spatial resolution (Karger et al. 2017).
Definition of climatically central and marginal populations
To distinguish ecologically marginal and central populations we first characterized the climatic niche of L. pomponium carrying out a principal component analysis (PCA) of the bioclimatic variables using the ‘ade4’ package implemented in R (R Core Team 2018). We considered “central” and “marginal” the populations falling into and outside the 70% of confidence ellipse, respectively. Then we grouped the marginal populations in different climate groups according to the PCA quadrants where they fall. Moreover, we tested differences among groups of populations because of mean annual temperature and annual precipitation using the non-parametric Kruskal–Wallis test. Kernel density plots were used to visualize the distribution of each variable.
Correlation between geographical and climatic distance
To test whether populations that are geographically peripheral are also ecologically different or marginal, we calculated the Euclidean distance from each population to the centroid of climatic space in the PCA and the Euclidean distance from each population to the centre of the distributional range. We calculated the correlation between the two distances using Spearman rank correlation coefficient.
Phenotypic variation
To assess whether different groups of populations differ in floral traits, we measured 562 flowers from 414 randomly chosen plants ranging from 8 to 34 per populations (according to population size) during the years 2017–2018 (250 and 312 flowers in 2017 and 2018, respectively). The number of flowers analysed per population ranged from 13 to 80 (Table 1 and Online Resource 1) and roughly 75% of measures were from single flowers on different plants. We analysed two traits involved in pollinator attraction: the number of flowers per scape and the corolla surface. In particular, corolla surface was calculated as the surface of an oblate spheroid (Fig. 2), measuring height and width of the corolla (the latter measured three times, one for each pair of tepals—CH and CW, respectively, in Fig. 2). We also analysed a trait involved in flower–pollinator interaction: the spatial separation of pollen presentation and pollen receipt; in particular, we measured the distance between the top of the ovary and the tip of the stigma (stigma position: AP in Fig. 2), the distance between the top of the ovary and the tip of the six stamens (anther position: AP in Fig. 2), and the length of the six anthers (AL in Fig. 2). We classified flowers in three categories: (1) flowers showing approach herkogamy, stigma above the anther; (2) flowers showing reverse herkogamy, stigma below the anther; and (3) flowers without herkogamy, stigma among the anthers. Floral measurements were obtained in field with a digital caliper (error = ± 0.01 mm).
Pollen limitation and reproductive performances
To test whether the self-fertilization rate was different between groups of populations, we bagged 183 flower buds from 139 plants (ranging from 10 to 20) in 12 populations using non-woven fabric bags. Furthermore, to test whether the degree of pollen limitation leading to reduced seed production was different between central and marginals populations, we assigned a total of 168 flowers from 143 plants (ranging from 3 to 28) in 17 populations to supplemental hand pollination (Ps) and 344 flowers from 279 plants (ranging from 4 to 42) in 17 populations to open pollination as control (Online Resource 1).
For each population, we quantified seed number on naturally pollinated flowers (Po) and supplementary-pollinated flowers (Ps) and thus obtained a value for pollen limitation (PL) using the formula of Baskin and Baskin (2017): PL = (Ps − Po)/Pmax [Ps or Po]. Values of PL range from 1 to − 1. Positive values indicate a lower seed set in natural than in pollen-supplemented flowers, negative values indicate a higher seed set in natural than in pollen-supplemented flowers. Because the number of pollen donors may affect the reproductive success (Schemske and Pautler 1984), we collected pollen from at least three donors.
To estimate Ps (seed set in pollen-supplemented flowers) and Po (seed set in open-pollinated flower), mature capsules were collected before dehiscence, preventing seed dispersion. Seeds were counted under a Leica M205 C stereomicroscope. We calculated seed set as filled seeds/total number of ovules (filled seeds + aborted seeds + unfertilized ovules).
Statistical analyses
To test whether the groups of populations differed significantly in number of flowers per scape, flower size, and pollen and ovules production, we applied the non-parametric Tukey–Kramer–Nemenyi post hoc test using the R ‘PCMMRplus’ package (Pohlert 2014), implemented in R (R Core Team 2018). To test whether the groups of populations differed significantly in the percentage of flowers belonging to different groups of populations, we used chi-squared or exact Fisher test when the expected frequencies was less than five in some cells.
Because seed production follows a binomial distribution, lacking the property of linearity and additivity, the effects of marginality of populations on plant fitness were analysed by fitting factorial generalized linear mixed models (GLMMS, logit link function, binomial distribution) to the seed set data with group of populations as fixed predictors, and populations as random factor. Statistical analyses were performed using the ‘lme4’ package (Bates et al. 2015) implemented in R (R Core Team 2018). Post hoc tests were conducted to evaluate pair-wise differences in measured traits between treatments using the ‘glht’ function in the ‘multicomp’ package (Hothorn et al. 2008) implemented in R (R Core Team 2018). Moreover, to test the relationship between elevation and flower size, number of flowers per scape, seed set and degree of pollen limitation, we calculated Spearman rank correlation coefficients.
Results
Central and marginal populations
The first two axes of the PCA explain 76.84% of the variation of the whole data set. The ellipses drawn include 70% of the data of the group. This allowed us to recognize five central populations (hereafter CC) and 15 marginal populations (Fig. 3). The marginal populations were further subdivided into three different groups growing under different climatic conditions (Fig. 4). The first group, hereafter called coastal marginal (CM) included four populations growing under warm and moist conditions, probably due to due to the vicinity to the sea (Figs. 3, 4). The second group, hereafter called inland marginal (IM) included five populations growing under warmest and driest conditions (Figs. 3, 4). The third group, hereafter called subalpine marginal (SM) included six populations of the subalpine belt growing under cold and wet conditions (Figs. 3, 4). Distance from the geographical centre and distance from the climatic centre were negatively correlated (ρ = − 0.17, p value = 0.48).
Principal component analysis of climate data for all known population locations of Lilium pomponium populations (grey dots). Ellipses include 70% of each class variance. Central populations are inside the ellipse while the marginal populations are outside. Numbers indicate the sampled populations (see Table 1). P01, P02, P03, P09: coastal marginal (CM); P04, P05, P06, P07, P08: inland marginal (IM); P10, P11, P12, P13, P18: central (CC); P14, P15, P16, P17, P19, P20: subalpine marginal (SM). Populations’ codes are those reported in Table 1. The nineteen bioclimatic variables (BIO01-BIO19) were downloaded from the CHELSA climate database website [https://chelsa-climate.org/]
Kernel density plots of average annual a temperature and b precipitation experienced by populations of Lilium pomponium in the four different sampled climatic conditions: CM coastal marginal populations, IM inland marginal populations, CC central population, and SM subalpine marginal populations. Different letters indicate statistical differences (i.e., P values ≤ 0.05). P values were calculated using the non-parametric Kruskal–Wallis test
Phenotypic variation
Flowers were significantly larger at low elevation in CM and IM (Fig. 5a); mean flower size decreased from 3.14 mm2 (CM), 3.03 (IM), 2.70 (CC) to 2.49 (SM) mm2. The number of flowers per scape was significantly lower in CM and CC (Fig. 5b). In particular, CM and CC plants bore 1.61 (sd = 1.07) and 2.12 (sd = 1.86) flowers, while SM and IM plants bore 2.89 (sd = 4.64) and 2.90 (sd = 2.06) flowers. In the majority of flowers (86%), the stigma was placed at the level of anthers (i.e., no herkogamy); 12% of flowers showed approach herkogamy and 2% of flowers showed reverse herkogamy. A significant difference in percentage of flowers with a separation between stigma and anthers was detected only between CM and the other groups (Fig. 5c). In particular, CM showed at the same time (Fig. 5c) the highest percentage of flowers with approach herkogamy (25.27%) and thus the lowest percentage of flowers without herkogamy (73.63%). Hence, flower size is significantly, positively correlated with elevation and approach herkogamy is particularly frequent in CM, where three out of four populations had a high proportion of flowers with marked approach herkogamy (Online Resource 2 Fig. S1a, c). In contrast, no correlation between the number of flowers per scape and elevation was detected (Online Resource 2 Fig. S1b).
Boxplot of corolla surface. a Flower size, b number of flowers per scape and c extent of herkogamy in populations of Lilium pomponium in four sampled climatic groups: coastal marginal populations (CM), inland populations (IM), central populations (CC), and subalpine marginal populations (SM). c Percentage of flowers with a separation between stigma and anthers. Different letters indicate statistical differences (i.e., P values ≤ 0.05). P values were calculated using non-parametric Tukey–Kramer–Nemenyi post hoc test to assess difference in flower size and number of flowers per scape and using chi-squared or exact Fisher test to assess difference in herkogamy
Pollen limitation and reproductive performances
No significant differences were detected in seed set among groups of populations. Seed set was pollen-limited mainly in CM (PL = 0.20) and in IM (PL = 0.12). In CC the mean value of PL was close to zero (PL = 0.005), suggesting no pollen limitation. Differently, the mean PL value of SM (Fig. 6) was weakly negative (PL = − 0.09). In the self-pollination treatment, only one out of the 93 flowers produced a fruit. Hence, seed set was not significantly correlated with altitude while the level of pollen limitation was significantly negatively correlated with altitude (Online Resource 2 Fig. S2a, b).
Comparisons of a seed set (mean ± 95% confidence interval) and b pollen limitation (mean ± standard error) in populations of Lilium pomponium in four sampled climatic groups: coastal marginal populations (CM), inland populations (IM), central populations (CC), and subalpine marginal populations (SM). Seed set was calculated as filled seeds/filled seeds + aborted seeds + unfertilized ovules and pollen limitation was calculated as: (Ps − Po)/Pmax [Ps or Po] where Po = seed number in naturally pollinated flowers and Ps seed number in supplementary-pollinated. Different letters indicate statistical differences (i.e., P values ≤ 0.05). P values were calculated using a general linear hypothesis tests
Discussion
Congruence between geographical and environmental marginality
According to the central–peripheral hypothesis (CPH), species are predicted to have higher performance in the centre of their distributional range where habitat conditions are expected to be more favourable and stable than in peripheral or marginal populations (Hengeveld and Haeck 1982; Brown 1984). In fact, the harsh environmental conditions at the periphery may result in a decline in pollinator service, such that populations at geographical and environmental edges are strongly pollen limited (Moeller et al. 2012), or in a decline in population size that causes less pollinator attraction (Hargreaves and Eckert 2014). In both cases, a reduction in seed set and an increase in pollen limitation occur due to a change in the pollination service in peripheral populations.
In L. pomponium, populations in marginal climatic groups CM and SM occur, respectively, at the southern and northern geographical extremes of the distributional range, while the marginal environmental group IM occurs closer to the centre of the distributional range than populations in the central group CC (squares and triangles in Fig. 1). In particular, environmentally marginal populations grow in warm and dry conditions (Fig. 4) and are located both at southern (i.e., CM group) and lower altitudinal (i.e., IM group) limits, while marginal populations growing in cold and wet conditions (i.e., SM group in Fig. 4) are located at highest altitude in the northern limit of the distributional range (Table 1 and Fig. 1). This result is in line with the lack of correlation between geographical and climatic distances (i.e. p value 0.19 and tau − 0.22), suggesting that populations near the geographical centre are not necessarily near the ecological centre of the species niche and vice versa. These results support the idea that geographical and environmental gradients are not necessarily concordant (Ribeiro and Fernandes 2000; Herlihy and Eckert 2005; Herrera and Bagaza 2008; Villellas et al. 2012; Pironon et al. 2015; Dallas et al. 2017) and that factors such as topography and environmental heterogeneity may impose marginal ecological conditions near the geographical centre (Soulé 1973; Doxa and Prastacos 2020).
Relationship between phenotypic and environmental marginality
Changes in the pollination environment due to changes in environmental conditions are expected to drive differentiation of floral traits between marginal and central populations. In particular, marginal populations are expected to diverge from central populations because they have smaller and fewer flowers, reduced stigma–anther separation, and high self-fertilization rate because of a reduction in pollinator visitation and less outcross pollination (Herlihy and Eckert 2005; Mimura and Aitken 2007). This pattern is not consistent among population groups in L. pomponium; the two environmentally marginal groups growing at the warm margin (i.e., the geographical peripheral CM and the geographical central IM) have similar traits and are rather different from central group. In particular, they diverge from CC by their pollinator-limited seed set (Fig. 6b and Online Resource 2 Fig. S2b) and significantly wider flowers (Fig. 5a and Online Resource 2 Fig. S1a). Moreover, CM populations have a higher percentage of flowers with approach herkogamy (Fig. 5c and Online Resource 2 Fig. S1c), i.e. a protruding stigma that reduces self-pollen deposition (Webb and Lloyd 1986) and IM has a high number of flowers per scape (Fig. 5b and Online Resource 2 Fig. S1b).
These results are congruent with the expectation that pollen limitation may select for enhanced attraction and favour large flowers that enhance visibility and, therefore, pollinator attraction (Thompson 2001; Arista and Ortiz 2007; Barrio and Teixido 2014) and favour the reliability of visits (Haig and Westoby 1988; Totland 2001; Teixido and Aizen 2019). Indeed, pollen-limited populations of L. pomponium have large flowers that may be favoured because they are more attractive to Lepidoptera (Thompson 2001), the main pollinators of L. pomponium (Casazza et al. 2018). Moreover, in the strongest pollen-limited group CM (PL = 0.20), the high percentage of flowers with a protruding stigma may contribute to limit self-pollen deposition that can cause self-interference that may reduce female fitness (Webb and Lloyd 1986; Li et al. 2013). In IM, the flowers are large and numerous (Fig. 5b and Online Resource 2 Fig. S1b), contrary to the general expectation of a trade-off between flower size and number because of energetic constraints (Sargent et al. 2007). Nevertheless, in this group, the high number of flowers per scape may be related to the small size of populations (Table 1). In fact, the high number of flowers may increase the frequency of within-plant pollinator movements favouring pollination of flowers by pollen from other flowers on the same plant (Mustajärvi et al. 2001; Iwaizumi and Sakai, 2004). In small-sized and pollen-limited populations (i.e., IM) of non-autogamous species, like L. pomponium (Casazza et al. 2018), this strategy may allow the production of a regular but low number of seeds (Roberts et al. 2014), even if it reduces outcrossing (Lloyd 1992; Harder and Barrett 1995).
In contrast, populations in the group growing in cold conditions (i.e., SM) are similar to those in the central group in that they show no evidence of pollinator limitation (Fig. 6b and Online Resource 2 Fig. Sb) and have small flowers, even though in SM they are numerous per scape (Fig. 5b Online Resource 2 Fig. S1b), according to the trade-off between flowers size and number. In SM the large number of flowers per scape might be a bet-hedging strategy to assure reproduction in unpredictable environments (Koops et al. 2003). In fact, despite their cost, the late-blooming flowers may act as a reserve when the earlier blooming ones are lost early in the season (Brown 1984), because of late spring frosts. Moreover, the seed set of SM is similar to that detected in pollen limited groups and fits with the observation that in cold environments low temperatures alone, or because they reduce pollinator activity, limit seed production (Totland 2001). In species with green photosynthetic fruits during seed maturation like L. pomponium, low temperatures may be particularly effective in constraining photosynthetic activity and thereby the amounts of resource allocated to seed development (Totland 2001). This result is in line with the idea that seed set may be limited by pollen receipt and/or resource availability (Haig and Westoby 1988). In fact, groups growing in warm conditions are limited more by pollen than resources and have large flowers. In contrast, the group growing in cold conditions is limited by resources and not by pollen and has small flowers.
Contrary to the general expectation of reduction of pollination in the environmentally marginal populations, in our study all groups have a quite similar and moderate seed set (ranging from 0.586 to 0.655, Fig. 6a and Online Resource 2 Fig. S2a). While the moderate seed set in marginal groups may be explained by pollen limitations or by an effect of low temperatures, the moderate seed set in the large populations of the central group (Table 1) may be explained by other non-mutually exclusive factors such as seed predation and herbivory (Garwood and Horvitz 1985; Knight et al. 2006; Straka and Starzomski 2015). For example, in groups growing in wetter and cool conditions like CC and in part also SM, seeds may be more prone to damages by the lily beetle (Lilioceris lilii Scopoli, 1763) that prefers shaded, cool and moist areas in mountain habitats (Majka and LeSage 2008). Moreover, the generally moderate seed set may be conditioned by the life-history strategy of L. pomponium; like other long-lived herbaceous perennials, the species may have a strategy of annually limited but inter-annually constant seed production, in which sub-maximal seed production is a part of a size-dependent strategy that maximises life-time seed production, without compromising adult survival (García and Zamora 2003; Andrieu et al. 2007).
Our results suggest that in L. pomponium not all environmental marginal groups differ from the central one, because local environmental condition resulting in an array of interaction among resource availability, biotic interactions and population size may differentially affect seed set and phenotypic variation in floral traits. The phenotypic variability in floral traits among populations in different environments may be due to plasticity—the ability of one genotype to alter its phenotype in response to environmental conditions—and/or genetic variation—an increase in the frequency of genotypes that have traits enhancing fitness. Our data were recorded in natural populations; hence, greenhouse experiment will be necessary to discriminate between these two non-exclusive possibilities.
Conclusion
The lack of separation between geographically peripheral and central groups in traits related to pollination environment and the occurrence of an environmental marginal group near the geographical centre are in line with the idea that CHP predictions are confirmed only when its assumptions are met (Lira-Noriega and Manthey 2014; Kennedy et al 2020). Our results suggest that variability in local conditions drives variation in floral traits and probably in the pollination environment. However, the differences in pollination environment related to marginal environments along a gradient is not a main determinant of the distribution limit of L. pomponium, as suggested by the similar seed set values recorded throughout the distributional range. In species with predominantly localised dispersal such as L. pomponium fine-scale landscape heterogeneity at the geographical periphery may influence population survival due to an inability to persist below a threshold of density (Keitt et al. 2001) irrespective of pollination success.
Availability of data and material
The datasets generated during the current study are available in the ZENODO digital repository, https://doi.org/10.5281/zenodo.3757739 and https://doi.org/10.5281/zenodo.3766851.
References
Aigner PA (2004) Floral specialization without trade-offs: optimal corolla flare in contrasting pollination environments. Ecology 85:2560–2569. https://doi.org/10.1890/03-0815
Andrieu E, Debussche M, Galloni M, Thompson JD (2007) The interplay of pollination, costs of reproduction and plant size in maternal fertility limitation in perennial Paeonia officinalis. Oecologia 152:515–524. https://doi.org/10.1007/s00442-007-0662-x
Arista M, Ortiz PL (2007) Differential gender selection on floral size: an experimental approach using Cistus salvifolius. J Ecol 95:973–982. https://doi.org/10.1111/j.1365-2745.2007.01276.x
Barrett SCH (2002) Sexual interference of the floral kind. Heredity 88:154–159. https://doi.org/10.1038/sj.hdy.6800020
Barrett SCH, Harder LD, Cole WW (2004) Correlated evolution of floral morphology and mating-type frequencies in a sexually polymorphic plant. Evolution 58:964–975
Barrio M, Teixido AL (2014) Sex-dependent selection on flower size in a large-flowered Mediterranean species: an experimental approach with Cistus ladanifer. Plant Syst Evol 301:113–124. https://doi.org/10.1007/s00606-014-1058-0
Baskin JM, Baskin CC (2017) Seed germination in cleistogamous species: theoretical considerations and a literature survey of experimental results. Seed Sci Res 27:84–98. https://doi.org/10.1017/s0960258517000058
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw. arXiv:1406.5823. https://doi.org/10.18637/jss.v067.i01
Berg RL (1960) The ecological significance of correlation pleiades. Evolution 14:171–180. https://doi.org/10.1111/j.1558-5646.1960.tb03076.x
Brown JH (1984) On the relationship between abundance and distribution of species. Am Nat 124:255–279. https://doi.org/10.1086/284267
Casazza G, Carta A, Giordani P, Guerrina M, Peruzzi L, Minuto L (2018) Reproductive biology of the threatened Lilium pomponium (Liliaceae), a species endemic to Maritime and Ligurian Alps. J Plant Res 131:633–640. https://doi.org/10.1007/s10265-018-1019-8
Dai W-K, Kadiori EL, Wang Q-F, Yang C-F (2017) Pollen limitation, plasticity in floral traits, and mixed mating system in an alpine plant Pedicularis siphonantha (Orobanchaceae) from different altitudes. J Syst Evol 55:192–199. https://doi.org/10.1111/jse.12240
Dallas T, Decker RR, Hastings A (2017) Species are not most abundant in the centre of their geographic range or climatic niche. Ecol Lett 20:1526–1533. https://doi.org/10.1111/ele.12860
Doxa A, Prastacos P (2020) Using Rao’s quadratic entropy to define environmental heterogeneity priority areas in the European Mediterranean biome. Biol Conserv. https://doi.org/10.1016/j.biocon.2019.108366
Eckert CG, Samis KE, Lougheed SC (2008) Genetic variation across species’ geographical ranges: the central–marginal hypothesis and beyond. Mol Ecol 17:1170–1188. https://doi.org/10.1111/j.1365-294X.2007.03659.x
Frazee JE, Marquis RJ (1994) Environmental contribution to floral trait variation in Chamaecrista fasciculata (Fabaceae: Caesalpinoideae). Am J Bot 81:206–215. https://doi.org/10.1002/j.1537-2197.1994.tb15431.x
Fryxell PA (1957) Mode of reproduction of higher plants. Bot Rev 23:135. https://doi.org/10.1007/BF02869758
Gamble DE, Bontrager M, Angert AL (2018) Floral trait variation and links to climate in the mixed-mating annual Clarkia pulchella. Botanique 96:425–435. https://doi.org/10.1139/cjb-2017-0234
García D, Zamora R (2003) Persistence, multiple demographic strategies and conservation in long-lived Mediterranean plants. J Veg Sci 14:921–926. https://doi.org/10.1111/j.1654-1103.2003.tb02227.x
Garwood NC, Horvitz CC (1985) Factors limiting fruit and seed production of a temperate shrub, Staphylea trifolia L. (Staphyleaceae). Am J Bot 72:453–466. https://doi.org/10.1002/j.1537-2197.1985.tb05369.x
Graae BJ, Vandvik V, Armbruster WS, Eiserhardt WL, Svenning J-C, Hylander K, Ehrlén J, Speed JDM, Klanderud K, Bråthen KA, Milbau A, Opedal ØH, Alsos IG, Rasmus Ejrnæs Bruun HH, Birks HJB, Westergaard KB, Birks HH, Lenoir J (2018) Stay or go—how topographic complexity influences alpine plant population and community responses to climate change. Perspect Plant Ecol 30:41–50. https://doi.org/10.1016/j.ppees.2017.09.008
Griffin PC, Willi Y (2014) Evolutionary shifts to self-fertilisation restricted to geographic range margins in North American Arabidopsis lyrata. Ecol Lett 17:484–490. https://doi.org/10.1111/ele.12248
Guo H, Mazer SJ, Du G (2010) Geographic variation in primary sex allocation per flower within and among 12 species of Pedicularis (Orobanchaceae): proportional male investment increases with elevation. Am J Bot 97:1334–1341. https://doi.org/10.3732/ajb.0900301
Haig D, Westoby M (1988) On limits to seed production. Am Nat 131:757–759. https://doi.org/10.1086/284817
Hampe A, Petit RJ (2005) Conserving biodiversity under climate change: the rear edge matters. Ecol Lett 8:461–467. https://doi.org/10.1111/j.1461-0248.2005.00739.x
Harder LD, Barrett SCH (1995) Mating cost of large floral displays in hermaphrodite plants. Nature 373:512–515. https://doi.org/10.1038/373512a0
Hargreaves AL, Eckert CG (2014) Evolution of dispersal and mating systems along geographic gradients: implications for shifting ranges. Funct Ecol 28:5–21. https://doi.org/10.1111/1365-2435.12170
Hargreaves AL, Jennifer LW, Eckert CG (2015) High-elevation range limit of an annual herb is neither caused nor reinforced by declining pollinator service. J Ecol 103:572–584. https://doi.org/10.1111/1365-2745.12377
Hegland SJ, Totland Ø (2005) Relationships between species’ floral traits and pollinator visitation in a temperate grassland. Oecologia 145:586–594. https://doi.org/10.1007/s00442-005-0165-6
Hengeveld R, Haeck J (1982) The distribution of abundance. I. Measurements. J Biogeogr 9:303–316. https://doi.org/10.2307/2844717
Herlihy CR, Eckert CG (2005) Evolution of self-fertilization at geographical range margins? A comparison of demographic, floral, and mating system variables in central vs. peripheral populations of Aquilegia canadensis (Ranunculaceae). Am J Bot 92:744–751. https://doi.org/10.3732/ajb.92.4.744
Herrera CM, Bazaga P (2008) Adding a third dimension to the edge of a species’ range: altitude and genetic structuring in mountainous landscapes. Heredity 100:275–285. https://doi.org/10.1038/sj.hdy.6801072
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. https://doi.org/10.1002/bimj.200810425
Iwaizumi MG, Sakai S (2004) Variation in flower biomass among nearby populations of Impatiens textori (Balsaminaceae): effects of population plant densities. Can J Bot 82:563–572. https://doi.org/10.1139/b04-013
Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW, Zimmermann NE, Linder HP, Kessler M (2017) Climatologies at high resolution for the earth’s land surface areas. Sci Data 4:170122. https://doi.org/10.1038/sdata.2017.122
Keitt TH, Lewis MA, Holt RD (2001) Allee effects invasion pinning and species borders. Am Nat 157:203–216. https://doi.org/10.1086/318633
Kennedy JP, Preziosi RF, Rowntree JK, Feller IC (2020) Is the central-marginal hypothesis a general rule? Evidence from three distributions of an expanding mangrove species, Avicennia germinans (L.) L. Mol Ecol 29:704–719. https://doi.org/10.1111/mec.15365
Knight TM, Steets JA, Ashman T-L (2006) A quantitative synthesis of pollen supplementation experiments highlights the contribution of resource reallocation to estimates of pollen limitation. Am J Bot 93:271–277. https://doi.org/10.3732/ajb.93.2.271
Koops MA, Hutchings JA, Adams BK (2003) Environmental predictability and the cost of imperfect information: influences on offspring size variability. Evol Ecol Res 5:29–42
Kropf M, Comes HP, Kadereit JW (2008) Causes of the genetic architecture of south-west European high mountain disjuncts. Plant Ecol Divers 1:217–228. https://doi.org/10.1080/17550870802331938
Kӧrner C (2003) The use of ‘altitude’ in ecological research. Trends Ecol Evol 22:569–574. https://doi.org/10.1016/j.tree.2007.09.006
Lambrecht SC, Morrow A, Hussey R (2017) Variation in and adaptive plasticity of flower size and drought-coping traits. Plant Ecol 218:647–660. https://doi.org/10.1007/s11258-017-0718-x
Lesica P, Allendorf FW (1995) When are peripheral populations valuable for conservation? Conserv Biol 9:753–760. https://doi.org/10.1046/j.1523-1739.1995.09040753.x
Lira-Noriega A, Manthey JD (2014) Relationship of genetic diversity and niche centrality: a survey and analysis. Evolution 68:1082–1093. https://doi.org/10.1111/evo.12343
Lloyd DG (1992) Self- and cross-fertilization in plants. II. The selection of self-fertilization. Int J Plant Sci 153:370–380. https://doi.org/10.1086/297041
Majka CG, LeSage L (2008) Introduced leaf beetles of the maritime provinces, 5: The Lily Leaf Beetle, Lilioceris lilii (Scopoli) (Coleoptera: Chrysomelidae). Proc Entomol Soc Wash 110:186–195. https://doi.org/10.4289/0013-8797-110.1.186
Mimura M, Aitken SN (2007) Increased selfing and decreased effective pollen donor number in peripheral relative to central populations in Picea sitchensis (Pinaceae). Am J Bot 94:991–998. https://doi.org/10.3732/ajb.94.6.991
Moeller DA, Geber MA, Eckhart VM, Tiffin P (2012) Reduced pollinator service and elevated pollen limitation at the geographic range limit of an annual plant. Ecology 93:1036–1048. https://doi.org/10.1890/11-1462.1
Mustajärvi K, Siikamaki P, Rytkonen S, Lammi A (2001) Consequences of plant population size and density for plant–pollinator interactions and plant performance. J Ecol 89:80–87. https://doi.org/10.1046/j.1365-2745.2001.00521.x
Papuga G, Gauthier P, Ramos J, Pons V, Pironon S, Farris E, Thompson JD (2015) Range-wide variation in the ecological niche and floral polymorphism of the western-Mediterranean geophyte Narcissus dubius Gouan. Int J Plant Sci 176:724–738. https://doi.org/10.1086/683010
Papuga G, Gauthier P, Pons V, Farris E, Thompson JD (2018) Ecological niche differentiation in peripheral populations: a comparative analysis of eleven Mediterranean plant species. Ecography 41:1–15. https://doi.org/10.1111/ecog.03331
Piñeiro R, Aguilar JF, Munt DD, Feliner GN (2007) Ecology matters: Atlantic–Mediterranean disjunction in the sand-dune shrub Armeria pungens (Plumbaginaceae). Mol Ecol 16:2155–2171. https://doi.org/10.1111/j.1365-294X.2007.03280.x
Pironon S, Villellas J, Morris WF, Doak DF, García MB (2015) Do geographic, climatic or historical ranges differentiate the performance of central versus peripheral populations? Glob Ecol Biogeogr 24:611–620. https://doi.org/10.1111/geb.12263
Pironon S, Papuga G, Villellas J, Angert AL, García MB, Thompson JD (2017) Geographic variation in genetic and demographic performance: new insights from an old biogeographical paradigm. Biol Rev 92:1877–1909. https://doi.org/10.1111/brv.12313
Pohlert T (2014) The pairwise multiple comparison of mean ranks package (PMCMR). R package
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Ribeiro KT, Fernandes GW (2000) Patterns of abundance of a narrow endemic species in a tropical and infertile montane habitat. Plant Ecol 147:205–217. https://doi.org/10.1023/a:1009883300536
Roberts DG, Ottewell KM, Whelan RJ, Ayre DJ (2014) Is the post-disturbance composition of a plant population determined by selection for outcrossed seedlings or by the composition of the seedbank? Heredity 112:409–414. https://doi.org/10.1038/hdy.2013.119
Sagarin RD, Gaines SD (2002) The ‘abundant centre’’ distribution: to what extent is it a biogeographical rule?’ Ecol Lett 5:137–147. https://doi.org/10.1046/j.1461-0248.2002.00297.x
Sargent RD, Goodwillie C, Kalisz S, Ree RH (2007) Phylogenetic evidence for a flower size and number trade-off. Am J Bot 94:2059–2062. https://doi.org/10.3732/ajb.94.12.2059
Schemske DW, Pautler L (1984) The effects of pollen composition on fitness components in a neotropical herb. Oecologia 62:31–36. https://doi.org/10.1007/BF00377369
Seguí J, Lázaro A, Traveset A, Salgado-Luarte C, Gianoli E (2018) Phenotypic and reproductive responses of an Andean violet to environmental variation across an elevational gradient. Alpine Bot 128:59–69. https://doi.org/10.1007/s00035-017-0195-9
Soulé M (1973) The epistasis cycle: a theory of marginal populations. Annu Rev Ecol Syst 4:165–187. https://doi.org/10.1146/annurev.es.04.110173.001121
Stone JL, Jenkins EG (2008) Pollinator abundance and pollen limitation of a solanaceous shrub at premontane and lower montane sites. Biotropica 40:55–61. https://doi.org/10.1111/j.1744-7429.2007.00339.x
Straka JR, Starzomski BM (2015) Fruitful factors: what limits seed production of flowering plants in the alpine? Oecologia 178:249–260. https://doi.org/10.1007/s00442-014-3169-2
Teixido AL, Aizen MA (2019) Reproductive assurance weakens pollinator-mediated selection on flower size in an annual mixed-mating species. Ann Bot Lond 123:1067–1077. https://doi.org/10.1093/aob/mcz014
Thompson JD (2001) How do visitation patterns vary among pollinators in relation to floral display and floral design in a generalist pollination system? Oecologia 126:386–394. https://doi.org/10.1007/s004420000531
Totland Ø (2001) Environment-dependent pollen limitation and selection on floral traits in an alpine species. Ecology 82:2233–2244. https://doi.org/10.1890/0012-9658(2001)082[2233:EDPLAS]2.0.CO;2
Villellas J, Ehrlén J, Olesen JM, Braza R, García MB (2012) Plant performance in central and northern peripheral populations of the widespread Plantago coronopus. Ecography 36:136–145. https://doi.org/10.1111/j.1600-0587.2012.07425.x
Vittoz P, Engler R (2007) Seed dispersal distances: a typology based on dispersal modes and plant traits. Bot Helv 117:109–124. https://doi.org/10.1007/s00035-007-0797-8
Webb CJ, Lloyd DG (1986) The avoidance of interference between the presentation of pollen and stigmas in angiosperms II. Herkogamy. N Z J Bot 24:163–178. https://doi.org/10.1080/0028825X.1986.10409726
Acknowledgements
The authors thank Sara Nicolini, Chiara Calise, Alessandro Rivata and Aleandra Di Caro for lab and field assistance.
Funding
Open access funding provided by Università degli Studi di Genova within the CRUI-CARE Agreement. This study was funded by the European Union’s Horizon 2020 research and innovation programme under Grant agreement no. 793226.
Author information
Authors and Affiliations
Contributions
CM and GC originally formulated the idea; CM, DD, LM and GC developed methodology and conducted fieldwork; CM and GC performed statistical analyses; CM, DD, LM, FM, JDT and GC wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Amy Parachnowitsch.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Macrì, C., Dagnino, D., Guerrina, M. et al. Effects of environmental heterogeneity on phenotypic variation of the endemic plant Lilium pomponium in the Maritime and Ligurian Alps. Oecologia 195, 93–103 (2021). https://doi.org/10.1007/s00442-020-04806-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-020-04806-6