Abstract
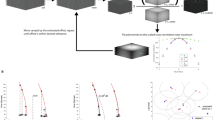
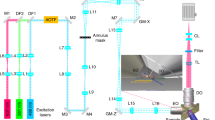
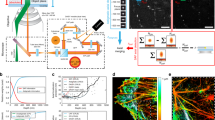
A key part of any super-resolution technique involves accurately correcting for mechanical motion of the sample and setup during acquisition. If left uncorrected, drift degrades the resolution of the final reconstructed image and can introduce unwanted artifacts. Here, we describe how to implement active stabilization, thereby reducing drift to ~1 nm across all three dimensions. In this protocol, we show how to implement our method on custom and standard microscopy hardware. We detail the construction of a separate illumination and detection path, dedicated exclusively to acquiring the diffraction pattern of fiducials deposited on the imaging slide. We also show how to focus lock and adjust the focus in arbitrary nanometer step size increments. Our real-time focus locking is based on kHz calculations performed using the graphics processing unit. The fast calculations allow for rapid repositioning of the sample, which reduces drift below the photon-limited localization precision. Our approach allows for a single-molecule and/or super-resolution image acquisition free from movement artifacts and eliminates the need for complex algorithms or hardware installations. The method is also useful for long acquisitions which span over hours or days, such as multicolor super resolution. Installation does not require specialist knowledge and can be implemented in standard biological laboratories. The full protocol can be implemented within ~2 weeks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The design for the bracket specific to the RM21 is available from GitHub (https://github.com/spcoelho/Active-Stabilization-Design). Microscope control and the Simulation and Hardware Test software is available from GitHub (https://github.com/spcoelho/Active-Stabilization.git). Example data for Figs. 1 and 5, and Supplementary Figs. 1 and 2 can be found on GitHub (https://github.com/spcoelho/Active-Stabilization-Test-Data) and image data can be found on the Zenodo online repository (https://doi.org/10.5281/zenodo.3973720 and https://doi.org/10.5281/zenodo.3974141).
Code availability
The active stabilization software, including the executables can be found on GitHub: https://github.com/spcoelho/Active-Stabilization.git
References
Hell, S. W. et al. The 2015 super-resolution microscopy roadmap. J. Phys. D. Appl. Phys. 48, 443001 (2015).
Betzig, E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 (2006).
Hess, S. T., Girirajan, T. P. & Mason, M. D. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys. J. 91, 4258–4272 (2006).
Rust, M. J., Bates, M. & Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3, 793–796 (2006).
Jungmann, R. et al. Multiplexed 3D cellular super-resolution imaging with DNA-PAINT and Exchange-PAINT. Nat. Methods 11, 313–318 (2014).
Nicovich, P. R., Owen, D. M. & Gaus, K. Turning single-molecule localization microscopy into a quantitative bioanalytical tool. Nat. Protoc. 12, 453–460 (2017).
Hell, S. W. & Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, 780–782 (1994).
Balzarotti, F. et al. Nanometer resolution imaging and tracking of fluorescent molecules with minimal photon fluxes. Science 355, 606–612 (2017).
Gustafsson, M. G. Nonlinear structured-illumination microscopy: wide-field fluorescence imaging with theoretically unlimited resolution. Proc. Natl Acad. Sci. USA 102, 13081–13086 (2005).
Hell, S. W. Far-field optical nanoscopy. Science 316, 1153–1158 (2007).
Pawley, J. Handbook of Biological Confocal Microscopy Vol. 236 (Springer Science & Business Media, 2006).
Schnitzbauer, J., Strauss, M. T., Schlichthaerle, T., Schueder, F. & Jungmann, R. Super-resolution microscopy with DNA-PAINT. Nat. Protoc. 12, 1198 (2017).
Deschout, H. et al. Precisely and accurately localizing single emitters in fluorescence microscopy. Nat. Methods 11, 253 (2014).
Moerner, W. E. New directions in single-molecule imaging and analysis. Proc. Natl Acad. Sci. USA 104, 12596–12602 (2007).
Coelho, S. et al. Ultraprecise single-molecule localization microscopy enables in situ distance measurements in intact cells. Sci. Adv. 6, eaay8271 (2020).
Huhle, A. et al. Camera-based three-dimensional real-time particle tracking at kHz rates and Ångström accuracy. Nat. Commun. 6, 1–8 (2015).
Fish, K. N. Total internal reflection fluorescence (TIRF) microscopy. Curr. Protoc. Cytom. 50, 12.18. 11–12.18. 13 (2009).
Kim, J. et al. Unveiling the relationship between the perovskite precursor solution and the resulting device performance. J. Am. Chem. Soc. 142, 6251–6260 (2020).
Tokunaga, M., Imamoto, N. & Sakata-Sogawa, K. Highly inclined thin illumination enables clear single-molecule imaging in cells. Nat. Methods 5, 159–161 (2008).
Huang, B., Wang, W., Bates, M. & Zhuang, X. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science 319, 810–813 (2008).
Pavani, S. R. P. et al. Three-dimensional, single-molecule fluorescence imaging beyond the diffraction limit by using a double-helix point spread function. Proc. Natl Acad. Sci. 106, 2995–2999 (2009).
Shechtman, Y., Sahl, S. J., Backer, A. S. & Moerner, W. Optimal point spread function design for 3D imaging. Phys. Rev. Lett. 113, 133902 (2014).
Shtengel, G. et al. Interferometric fluorescent super-resolution microscopy resolves 3D cellular ultrastructure. Proc. Natl Acad. Sci. 106, 3125–3130 (2009).
Shroff, H., Galbraith, C. G., Galbraith, J. A. & Betzig, E. Live-cell photoactivated localization microscopy of nanoscale adhesion dynamics. Nat. Methods 5, 417 (2008).
Diekmann, R. et al. Chip-based wide field-of-view nanoscopy. Nat. Photonics 11, 322–328 (2017).
Huang, F. et al. Ultra-high resolution 3D imaging of whole cells. Cell 166, 1028–1040 (2016).
Gao, L., Shao, L., Chen, B.-C. & Betzig, E. 3D live fluorescence imaging of cellular dynamics using Bessel beam plane illumination microscopy. Nat. Protoc. 9, 1083–1101 (2014).
Baek, J. et al. Imaging galectin-3 dependent endocytosis with lattice light-sheet microscopy. SPIE Proceedings Vol. 10340 (eds. Sampson, D. D. et al.) (SPIE, 2017).
Aoki, K. & Matsuda, M. Visualization of small GTPase activity with fluorescence resonance energy transfer-based biosensors. Nat. Protoc. 4, 1623 (2009).
Poland, S. P. et al. A high speed multifocal multiphoton fluorescence lifetime imaging microscope for live-cell FRET imaging. Biomed. Opt. Express 6, 277–296 (2015).
Poland, S. P. et al. Time-resolved multifocal multiphoton microscope for high speed FRET imaging in vivo. Opt. Lett. 39, 6013–6016 (2014).
Suhling, K. et al. Fluorescence lifetime imaging (FLIM): Basic concepts and some recent developments. Med. Photonics 27, 3–40 (2015).
Suhling, K. et al. in Handbook of Photonics for Biomedical Engineering 353–405 (Springer, 2017).
Suhling, K. et al. in Advanced Time-Correlated Single Photon Counting Applications 119–188 (Springer, 2015).
Burke, D., Patton, B., Huang, F., Bewersdorf, J. & Booth, M. J. Adaptive optics correction of specimen-induced aberrations in single-molecule switching microscopy. Optica 2, 177–185 (2015).
Coelho, S. et al. Multifocal multiphoton microscopy with adaptive optical correction. SPIE Proceedings Vol. 8588 (SPIE, 2013).
Coelho, S., Poland, S. P., Devauges, V. & Ameer-Beg, S. M. Adaptive optics for a time-resolved Förster resonance energy transfer (FRET) and fluorescence lifetime imaging microscopy (FLIM) in vivo. Opt. Lett. 45, 2732–2735 (2020).
Eggeling, C. et al. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 457, 1159–1162 (2009).
Boutros, M., Heigwer, F. & Laufer, C. Microscopy-based high-content screening. Cell 163, 1314–1325 (2015).
Gustavsson, A.-K., Petrov, P. N., Lee, M. Y., Shechtman, Y. & Moerner, W. 3D single-molecule super-resolution microscopy with a tilted light sheet. Nat. Commun. 9, 1–8 (2018).
Giessibl, F. J. Advances in atomic force microscopy. Rev. Mod. Phys. 75, 949 (2003).
Schmidt, P. D., Reichert, B. H., Lajoie, J. G. & Sivasankar, S. Method for high frequency tracking and sub-nm sample stabilization in single molecule fluorescence microscopy. Sci. Rep. 8, 13912 (2018).
Balinovic, A., Albrecht, D. & Endesfelder, U. Spectrally red-shifted fluorescent fiducial markers for optimal drift correction in localization microscopy. J. Phys. D. Appl. Phys. 52, 204002 (2019).
Henriques, R. et al. QuickPALM: 3D real-time photoactivation nanoscopy image processing in ImageJ. Nat. Methods 7, 339–340 (2010).
Wang, Y. et al. Localization events-based sample drift correction for localization microscopy with redundant cross-correlation algorithm. Opt. Express 22, 15982–15991 (2014).
Pertsinidis, A., Zhang, Y. & Chu, S. Subnanometre single-molecule localization, registration and distance measurements. Nature 466, 647–651 (2010).
McGorty, R., Kamiyama, D. & Huang, B. Active microscope stabilization in three dimensions using image correlation. Opt. Nanoscopy 2, 3 (2013).
Grover, G., Mohrman, W. & Piestun, R. Real-time adaptive drift correction for super-resolution localization microscopy. Opt. Express 23, 23887–23898 (2015).
Ma, H., Xu, J., Jin, J., Huang, Y. & Liu, Y. A simple marker-assisted 3D nanometer drift correction method for superresolution microscopy. Biophys. J. 112, 2196–2208 (2017).
Gosse, C. & Croquette, V. Magnetic tweezers: micromanipulation and force measurement at the molecular level. Biophys. J. 82, 3314–3329 (2002).
van Loenhout, M. T. J., Kerssemakers, J. W. J., De Vlaminck, I. & Dekker, C. Non-bias-limited tracking of spherical particles, enabling nanometer resolution at low magnification. Biophys. J. 102, 2362–2371 (2012).
Chivers, C. E., Koner, A. L., Lowe, E. D. & Howarth, M. How the biotin–streptavidin interaction was made even stronger: investigation via crystallography and a chimaeric tetramer. Biochem. J. 435, 55–63 (2011).
Haber, C. & Wirtz, D. Magnetic tweezers for DNA micromanipulation. Rev. Sci. Instrum. 71, 4561–4570 (2000).
Bormuth, V. et al. Optical trapping of coated microspheres. Opt. Express 16, 13831–13844 (2008).
Huang, B., Jones, S. A., Brandenburg, B. & Zhuang, X. Whole-cell 3D STORM reveals interactions between cellular structures with nanometer-scale resolution. Nat. Methods 5, 1047–1052 (2008).
van de Linde, S. Single-molecule localization microscopy analysis with ImageJ. J. Phys. D. Appl. Phys. 52, 203002 (2019).
Acknowledgements
We are grateful for support from the Australia Research Council (CE140100011 to K.G., FL150100060 and CE140100036 to J.J.G.) and National Health and Medical Research Council of Australia (APP1059278 to K.G.). We also thank L. Lee and Y. Gambin for useful discussions; M. Catarino, M. Farrell, and J. Goyette for assistance with the manuscript preparation; and M. Farrell for help with software troubleshooting.
Author information
Authors and Affiliations
Contributions
S.C. and J.B. designed and implemented the active stabilization, built the original Feedback SMLM, and performed the experiments. S.C. and J.W. implemented active stabilization on the Nikon Ti. J.W. implemented active stabilization custom microscope units. S.C., J.J.G., and K.G. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Gerhard Schütz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol Coelho, S. et al. Sci. Adv. 6, eaay8271 (2020): https://advances.sciencemag.org/content/6/16/eaay8271
Supplementary information
Supplementary Information
Supplementary Figs. 1–3.
Rights and permissions
About this article
Cite this article
Coelho, S., Baek, J., Walsh, J. et al. 3D active stabilization for single-molecule imaging. Nat Protoc 16, 497–515 (2021). https://doi.org/10.1038/s41596-020-00426-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-00426-9
This article is cited by
-
Direct-laser writing for subnanometer focusing and single-molecule imaging
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.