- 1Telethon Kids Institute, University of Western Australia, Nedlands, WA, Australia
- 2INRA Pays de la Loire, UR 1268 Biopolymers Interactions Assemblies (BIA) Nantes, Nantes, France
- 3Centre de recherche de I‘Institut de Cardiologie et de Pneumologie de Québec, Université, Laval, QC, Canada
- 4College of Science, Health, Engineering and Education, Murdoch University, Perth, WA, Australia
- 5Child Health Research Centre, The University of Queensland, Brisbane, QLD, Australia
We recently reported that offspring of mice treated during pregnancy with the microbial-derived immunomodulator OM-85 manifest striking resistance to allergic airways inflammation, and localized the potential treatment target to fetal conventional dendritic cell (cDC) progenitors. Here, we profile maternal OM-85 treatment-associated transcriptomic signatures in fetal bone marrow, and identify a series of immunometabolic pathways which provide essential metabolites for accelerated myelopoiesis. Additionally, the cDC progenitor compartment displayed treatment-associated activation of the XBP1-ERN1 signalling axis which has been shown to be crucial for tissue survival of cDC, particularly within the lungs. Our forerunner studies indicate uniquely rapid turnover of airway mucosal cDCs at baseline, with further large-scale upregulation of population dynamics during aeroallergen and/or pathogen challenge. We suggest that enhanced capacity for XBP1-ERN1-dependent cDC survival within the airway mucosal tissue microenvironment may be a crucial element of OM-85-mediated transplacental innate immune training which results in postnatal resistance to airway inflammatory disease.
Introduction
The neonatal period represents a time of high risk for infection-related morbidity/mortality resulting from the combined effects of maturational deficiencies in both anti-microbial defense mechanisms that mediate pathogen recognition and elimination, and in the accompanying regulatory mechanisms required for calibration of these responses to minimize inflammatory damage to host tissues (1, 2). With respect specifically to the lung tissue microenvironment, a crucial factor governing the kinetics of postnatal acquisition of immune competence is the rate of development of the airway mucosal cDC network which controls local immune surveillance (3, 4).
In addition to increased susceptibility to infectious diseases, the seeds for development of a range of non-communicable diseases exemplified by asthma and aero-allergies are also frequently sewn during this early postnatal window period (5), suggesting that maturational deficiencies in immune function(s) may also be risk factors in this context. In this regard, maternal immune perturbations have been acknowledged to significantly influence fetal immune development, but the underlying mechanisms remain poorly characterized (6). Epidemiological data from studies on traditional farming families in Europe and USA suggesting that benign environmental microbial exposures of mothers during pregnancy can promote prenatal immune maturation within their offspring, leading to reduced susceptibility to postnatal development of respiratory inflammatory diseases (7, 8), have stimulated wide-spread interest in this issue. This capacity for microbial exposures to modulate immune development is consistent with the paradigm of “immune training”, whereby exposure to certain classes of microbial stimuli can alter the long-term functional state of innate immune cells, occurring at the progenitor level in the bone marrow (BM) (9–11), leading to optimized peripheral immune responsiveness to other unrelated microorganisms (12). With this in mind, there is growing interest in the concept that immune training can be therapeutically harnessed (13), particularly during prenatal development, to enhance immunocompetence within the offspring (14).
We recently reported that oral treatment of pregnant mice with the microbial-derived immunomodulator OM-85 reduces susceptibility of their offspring to the development of Th2-driven allergic airways inflammation, and identified myeloid progenitors in the offspring BM (which supply precursor DC to eventually populate mucosal DC networks) as a major target for maternal treatment effects (15). In the study presented here, we employed transcriptomic profiling to characterize gene networks activated in fetal BM (fBM) as a result of maternal OM-85 treatment, and identify the principal treatment targets as immunometabolic pathways supplying cellular cholesterol essential for rapid expansion of myeloid precursor compartments, and which have previously been recognized as hallmarks of classical immune training-associated gene signatures. We additionally identify activation of the XBP1-ERN1 signalling axis in the cDC precursor compartment, which has previously been associated with survival-under-stress, especially within the lung mucosal microenvironment.
Methods
Animals
Specific pathogen-free BALB/c mice were purchased from the Animal Resource Centre (Murdoch, Western Australia, Australia). All mice were housed under specific pathogen-free conditions at the Telethon Kids Institute Bioresources Centre.
Time-Mated Pregnancies
Female BALB/c mice 8–12 weeks of age were time-mated with male BALB/c studs 8–26 weeks of age. Male studs were housed individually with 1–2 females overnight. The detection of a vaginal plug the following morning was designated gestation day (GD) 0.5.
Maternal OM-85 Treatment
OM-85 (OM Pharma) is an endotoxin-low lyophilized extract containing a cocktail of TLR ligands derived from 8 major respiratory tract bacterial pathogens (Haemophilus influenzae, Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus viridians, Klebsiella pneumoniae, Klebsiella ozaenae, Staphylococcus aureus and Neisseria catarrhalis) (16, 17). Based on previously optimized dosing concentrations (18, 19), time-mated pregnant BALB/c mice received daily oral feeding of lyophilized OM-85 reconstituted in phosphate-buffered saline (PBS; prepared in-house) via pipette at a concentration of 400mg/kg body weight for the second half of gestation (GD9.5 – 17.5). Control pregnant mice were left untreated for the duration of the study, as based on our previously published studies which demonstrated no difference between PBS (vehicle)-treated and untreated mothers (18). All pregnant mice were handled equivalently throughout the duration of the study. All maternal treatment was performed with a single batch of OM-85 (batch# 1812162).
Tissue Collection
Pregnant BALB/c mice were sacrificed 24 h after the final OM-85 dose at GD18.5. Both horns of the uterus were removed and fetuses sacrificed by decapitation. Fetal hind legs (cleaned of excess tissue) were removed and long bones (femur and tibia) collected. Fetal long bone samples for flow cytometry were collected into cold PBS + 0.1% bovine serum albumin (BSA) and stored on ice. Fetal long bone samples for transcriptomic analysis were collected into RNAlater® stabilization solution (Sigma-Aldrich). Samples collected into RNAlater® were stored overnight at 4°C, then transferred to 1.5ml Eppendorf tubes (Eppendorf) and frozen at −80°C for future transcriptome profiling. All fetal samples were kept as individuals and not pooled. Dead fetuses were excluded from the study.
Single-Cell Suspension Preparation
Fetal long bones were prepared by mincing with a scalpel followed by enzymatic digestion, as previously detailed (15). Briefly, minced bones were resuspended in 10ml GKN (11mM D-glucose, 5.5mM KCl, 137mM NaCl, 25mM Na2HPO4; prepared in-house) + 10% fetal calf serum (FCS; Serana) with collagenase IV (Worthington Biochemical Corp.) and DNase (Sigma-Aldrich) at 37°C under gentle agitation for 60 min. Digested whole bone homogenates were filtered through sterile cotton wool columns (5ml syringe containing cotton wool; prepared in-house) coated with FCS to remove debris, centrifuged and resuspended in cold PBS for total cell counts.
Flow Cytometry
Fetal whole bone single-cell suspensions (prepared above) were used for all immunostaining. Panels of monoclonal antibodies (purchased from BD Biosciences unless otherwise stated) were developed to enable phenotypic characterization of committed myeloid cells: CD3-FITC (clone 17A2), CD11b-BV510 (clone M1/70), CD11c-BV711 (clone HL3), CD19-APC-H7 (clone 1D3), Gr-1-Biotin (clone RB6-8C5), CD45R/B220-PerCP-Cy5.5 (clone RA3-6B2) NKp46-PE-Cy7 (clone 29A1.4; BioLegend), SIRPα-APC (clone P84; BioLegend), I-A/I-E-BV421 (clone M5/114.15.2; BioLegend), F4/80-BV785 (clone BM8; BioLegend), Viability-AF700, Streptavidin-BV605; hematopoietic stem and progenitor cells: CD2-Biotin (clone RM2-5), CD3-Biotin (clone 145-2C11), CD4-Biotin (clone GK1.5), CD5-Biotin (clone 53-7.3), CD8α-Biotin (clone 53-6.7), CD19-Biotin (clone 1D3), CD45R/B220-Biotin (RA3-6B2), Gr-1-Biotin (clone RB6-8C5), Ter119-Biotin (clone TER-119), CD16/32-PerCP-Cy5.5 (clone 2.4G2), CD34-FITC (clone RAM34), IL-7Rα-PE-Cy7 (clone SB/199), Flt-3-PE (clone A2F10.1), c-Kit-APC-Cy7 (clone 2B8), Sca-1-BV510 (clone D7), CX3CR1-APC (clone SA011F11; BioLegend), NKG2D-BV711 (clone CX5), Viability-AF700, Streptavidin-BV605 and XBP1s-expressing bone marrow cells: CD3-FITC (clone 17A2), CD11b-BV510 (clone M1/70), CD11c-AF700 (clone HL3), CD19-APC-H7 (clone 1D3), I-A/I-E-AF647 (clone M5/114.15.2), CD45R/B220-PE-CF594 (RA3-6B2), Gr-1-Biotin (clone RB6-6B2), NKp46-PE-Cy7 (clone 29A1.4; BioLegend), F4/80-BV785 (clone BM8; BioLegend), XBP1s-BV421 (clone Q3-695), Streptavidin-BV605. Intracellular staining for XBP1s was performed using an intracellular Foxp3/Transcription factor staining buffer kit (eBioscience). Data acquisition was performed on a 4-laser LSRFortessa (BD Bioscience). All samples were kept as individuals and not pooled. Immune cell phenotypic characterization was performed using FlowJo software (version 10.1, Tree Star). Fluorescence minus one (FMO) staining controls were used for all panels where necessary (Supplementary Figure 1). Flow cytometry data quality was based on primary time gates to ensure appropriate laser delay (pre-determined by automated CS&T) during sample acquisition.
Flow Cytometric Statistical Analyses
Statistical analysis and graphing was performed using GraphPad Prism (GraphPad software; version 7.0a). Statistical significance of p<0.05 was considered significant. Unpaired, two-tailed Student’s t-test or Mann Whitney U test were used based on distribution of the data as determined by D’Agostino-Pearson omnibus normality test. Internal correlation within the untreated and OM-85 treated groups was assessed by controlling for family clustering using Generalized Estimation Equation (20) or Wilcoxon Rank-Based Test for Clustered Data (arXiv:1706.03409) as based on distribution of the data. Significance of the findings were not influenced by family clustering. Part of the cDC flow cytometry data presented in Figure 1 and MDP data presented in Figure 2 has been published in a forerunner manuscript (15).
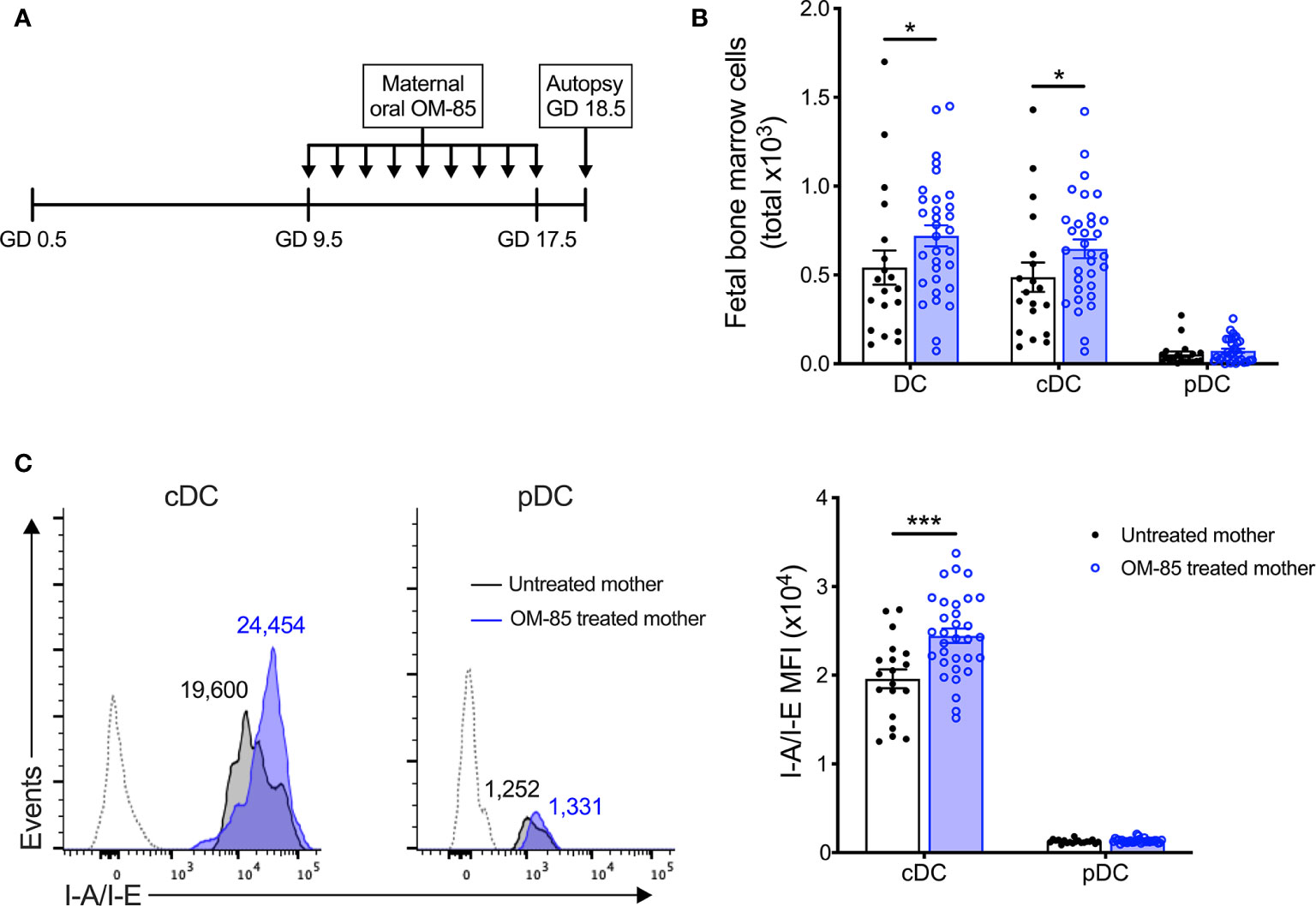
Figure 1 Maternal OM-85 treatment during pregnancy selectively modulates the fetal bone marrow conventional DC subset. (A) Kinetics of maternal OM-85 treatment beginning at GD 9.5 with daily oral treatment until GD 17.5 and autopsy 24 h post final treatment at GD 18.5. (B) Absolute numbers of DC, cDC and pDC in BM of fetuses from OM-85-treated and untreated mothers. (C) Mean fluorescence intensity (MFI) of I-A/I-E expression on cDC and pDC in fBM. Dotted histograms indicate FMO staining controls. Numbers above plot indicate representative MFI of I-A/I-E. Data are presented from individual animals comparing fetuses from OM-85-treated and untreated mothers and displayed as bar graphs showing mean ± SEM of n = 8 independent experiments, with each experiment containing fetuses from 1 untreated and 1 OM-85 treated mother. Statistical significance was determined using Mann-Whitney U test (B) or Student’s t test (C) based on distribution of the data as determined by D’Agostino-Pearson omnibus normality test. *p < 0.05, ***p < 0.001.
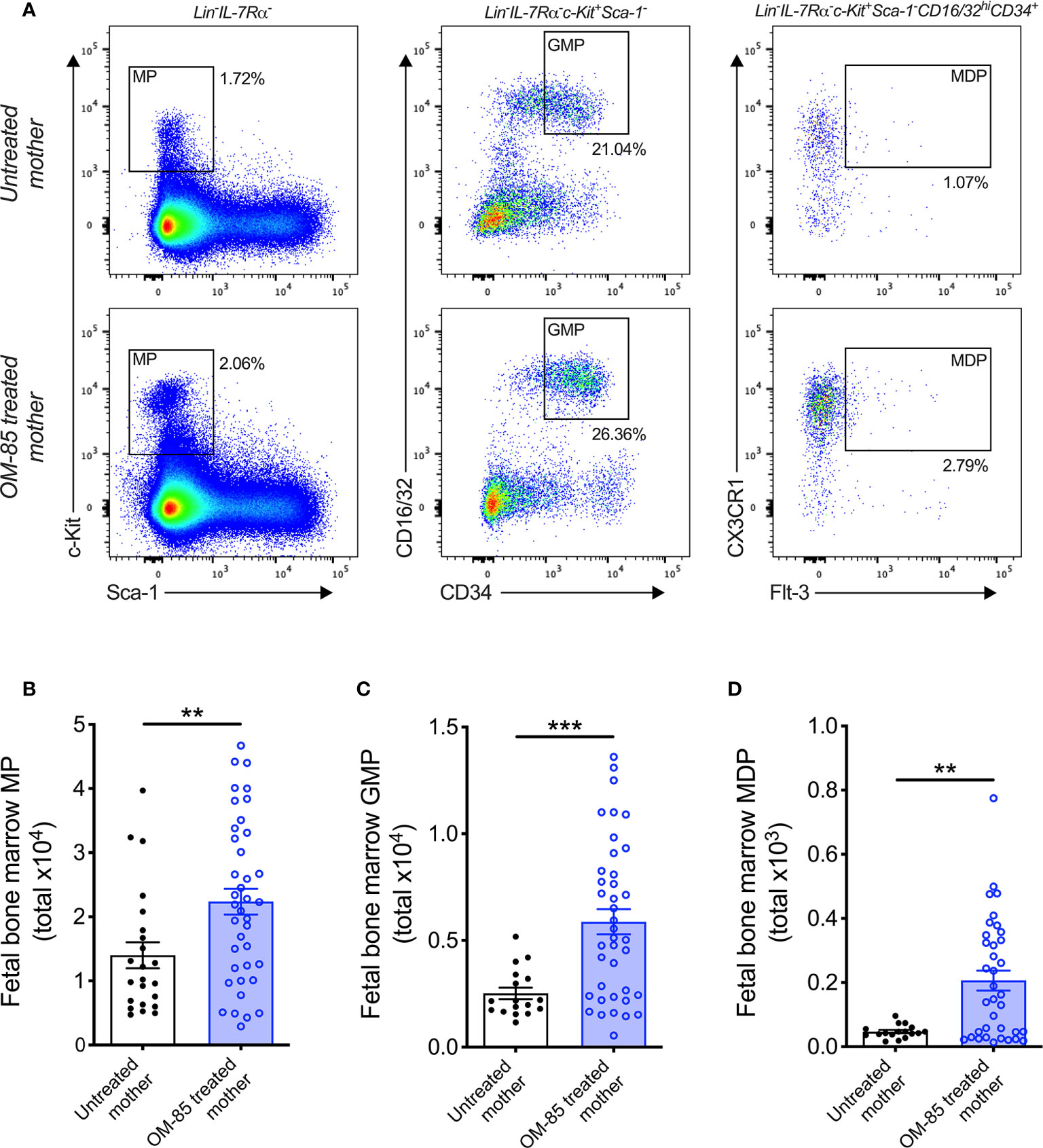
Figure 2 Treatment of mothers with OM-85 during pregnancy boosts myeloid progenitor subsets within fetal bone marrow. (A) Representative flow cytometry plots for the identification of MP, GMP and MDP within fBM. Absolute numbers of (B) MP, (C) GMP and (D) MDP in BM of fetuses from OM-85-treated and untreated mothers. Data are presented from individual animals comparing fetuses from OM-85-treated and untreated mothers and displayed as bar graphs showing mean ± SEM of n = 8 independent experiments, with each experiment containing fetuses from 1 untreated and 1 OM-85 treated mother. Statistical significance was determined using Student’s t test (C) or Mann-Whitney U test (B, D) based on distribution of the data as determined by D’Agostino-Pearson omnibus normality test. **p < 0.01, ***p < 0.001.
Transcriptome Profiling (RNA-Seq)
Tissue Preparation, RNA Extraction, and Transcriptome Profiling
Fetal bone marrow samples were homogenized using a rotor-star homogenizer (Qiagen) and total RNA extracted via TRIzol (Invitrogen), followed by clean-up using RNeasy MinElute Cleanup Kit (Qiagen). RNA integrity was determined using an Agilent 2100 Bioanalyzer [Agilent Technologies; RIN: 10 ± 0 (mean ± SD)]. One microgram total RNA (n = 32) was shipped on dry ice to the Australia Genome Research Facility (AGRF) for library preparation (TruSeq Stranded mRNA Library Prep Kit, Illumina) and sequencing (Illumina HiSeq2500, 50-bp singe-end reads, v4 chemistry).
RNA-Seq Data Analysis
Pre-Processing and Exploratory Data Analysis
RNA-seq data was analysed in the R environment for statistical computing. Sequencing data quality control (QC) was performed with the Bioconductor package Rqc (21). Sequencing reads were aligned to the reference murine genome (mm10) using Subread and summarized at the gene level using featureCounts (22). Genes with <500 total counts across the data were removed from the analysis. Sample QC was performed by analysing the distribution of the raw read counts to check for sample outliers using boxplots, relative log-transformed expression (RLE) plots and principal component analysis (PCA), before and after global-scale median normalization. Differential expression analysis: Differentially expressed genes (DEG) were identified employing the DESeq2 package (23). DESeq2 utilizes a negative binomial distribution model, with a False Discovery Rate adjusted P-value for multiple comparisons. Genes were deemed significant with an adjusted P-value < 0.1. Pathways analysis: Pathways enrichment analysis was performed using the InnateDB database (24) with Benjamini & Hochberg adjusted P-value ≤ 0.05 deemed significant. Upstream regulator analysis: Ingenuity Systems Upstream Regulator Analysis (25) was employed to identify putative molecular drives of the DEG patterns. Significance was determined by activation Z-score ≥ 2 and P-value of overlap ≤ 0.05.
Study Approval
All animal experiments were formally approved by the Telethon Kids Institute Animal Ethics Committee, operating under the guidelines developed by the National Health and Medical Research Council of Australia for the care and use of animals in scientific research.
Results
Maternal OM-85 Treatment Selectively Accelerates Functional Maturation of cDCs in Fetal Bone Marrow
To elucidate the mechanisms-of-action of maternal OM-85 treatment, we examined the in utero fetal response at gestation day (GD) 18.5 (Figure 1A), 2 days prior to expected natural term delivery. Primary observations identified a significant increase in the cellularity of fBM following maternal OM-85 treatment as compared to fBM from untreated mothers (data not shown). Targeted phenotypic analysis of the fBM myeloid compartment using multicolor flow cytometry (Supplementary Figure 2) revealed significant expansion of the total dendritic cell (DC) pool in fetuses from OM-85 treated mothers as compared to equivalent fetal samples from untreated mothers (Figure 1B). Further characterization of the BM DC response demonstrated that this increase was restricted to the CD11b+B220-CD11c+Gr-1-SIRPα+I-A/I-E+ conventional DC (cDC) subset as previously described (15), with no parallel changes observed in CD11b-B220+CD11c+Gr-1+I-A/I-E+ plasmacytoid DC (pDC) (Figure 1B). These cDC-specific changes in fBM mirror our recent observations of increased cDC yields from BM cultures and peripheral lung from the offspring of OM-85-treated mothers in the early postnatal period (15). We next turned our attention to fetal DC maturation state as determined by surface I-A/I-E expression. As shown in Figure 1C, maternal OM-85 treatment for the last half of gestation enhanced I-A/I-E expression on cDC in fBM when compared to fBM cDC from untreated mothers. Collectively, these observations suggest that transplacental signals generated at the feto-maternal interface following OM-85 treatment during pregnancy can “train” the developing fetal immune system via promoting the development of a fBM cDC compartment exhibiting a phenotype associated with enhanced functional competence.
Expansion of Fetal Bone Marrow Myeloid Progenitor Subsets Following Maternal OM-85 Treatment
Previous studies from our laboratory have additionally identified postnatal expansion of BM myeloid progenitor (MP) cell populations as an effect of maternal treatment with OM-85 during pregnancy (15). These findings mirror that of recent studies which identified modulation of BM MP as an important component of conventional immune training mediated by both β-glucan (10, 26) and Bacillus Calmette-Guérin (BCG) (11). Based on these findings, we hypothesized that the enhanced cDC population within fBM following maternal OM-85 treatment would also be accompanied by concomitant upregulation of upstream MP subsets. Although the linear commitment model of myeloid progenitor subsets remains controversial (27–29), using this approach for our hierarchical flow cytometric analysis of fBM (Figure 2A; Supplementary Figure 3) demonstrated a significant increase in total Lin-IL-7Rα-c-Kit+Sca-1- MP (Figure 2B), Lin-IL-7Rα-c-Kit+Sca-1-CD16/32hiCD34+ granulocyte-macrophage progenitor (GMP; Figure 2C) (30, 31) and Lin-IL-7Rα-c-Kit+Sca-1-CD16/32hiCD34+CX3CR1+Flt-3+ macrophage-dendritic cell progenitor (MDP; Figure 2D) (32, 33) populations within the BM compartment following maternal OM-85 treatment, when compared to fBM from untreated mothers. However, no changes were observed in the Lin-IL-7Rα+c-Kit+Sca-1+Flt-3+ common lymphoid progenitor (CLP) (34, 35) or Lin-IL-7Rα+c-Kit+Sca-1+NKG2D+ pre-natural killer cell progenitor (pre-NKp) (36, 37) populations following maternal OM-85 treatment (Supplementary Figure 4). Consistent with the findings in Figures 1B, C, these data provide further evidence that maternal OM-85 treatment selectively modulates the offspring BM myeloid lineage in utero, beginning at the early-stage myeloid progenitor level through to the terminal cDC populations which are responsible for seeding peripheral tissues during early postnatal life to provide local DC-mediated immune surveillance.
Maternal OM-85 Treatment Activates Key Regulators of the UPR Pathway in Fetal Bone Marrow
To gain further insight into the molecular mechanisms underpinning the maternal OM-85-treatment effects, we employed transcriptomic profiling of fBM cells. Comparison of the transcriptomic profiles in the treated versus untreated groups indicated that maternal OM-85 treatment resulted in 152 differentially expressed genes (DEG) in fBM (119 upregulated, 33 downregulated; Figure 3A, Supplementary Table 1). We then interrogated the DEG for enrichment of biological pathways employing InnateDB (24), focusing on the upregulated DEG response given the limited number of downregulated DEG identified. In fBM, upregulated DEG were enriched for genes involved in multiple aspects of protein metabolism, the endoplasmic reticulum (ER) stress response, the unfolded protein response (UPR), cholesterol biosynthesis and lipid metabolism (Figure 3B; Supplementary Table 2).
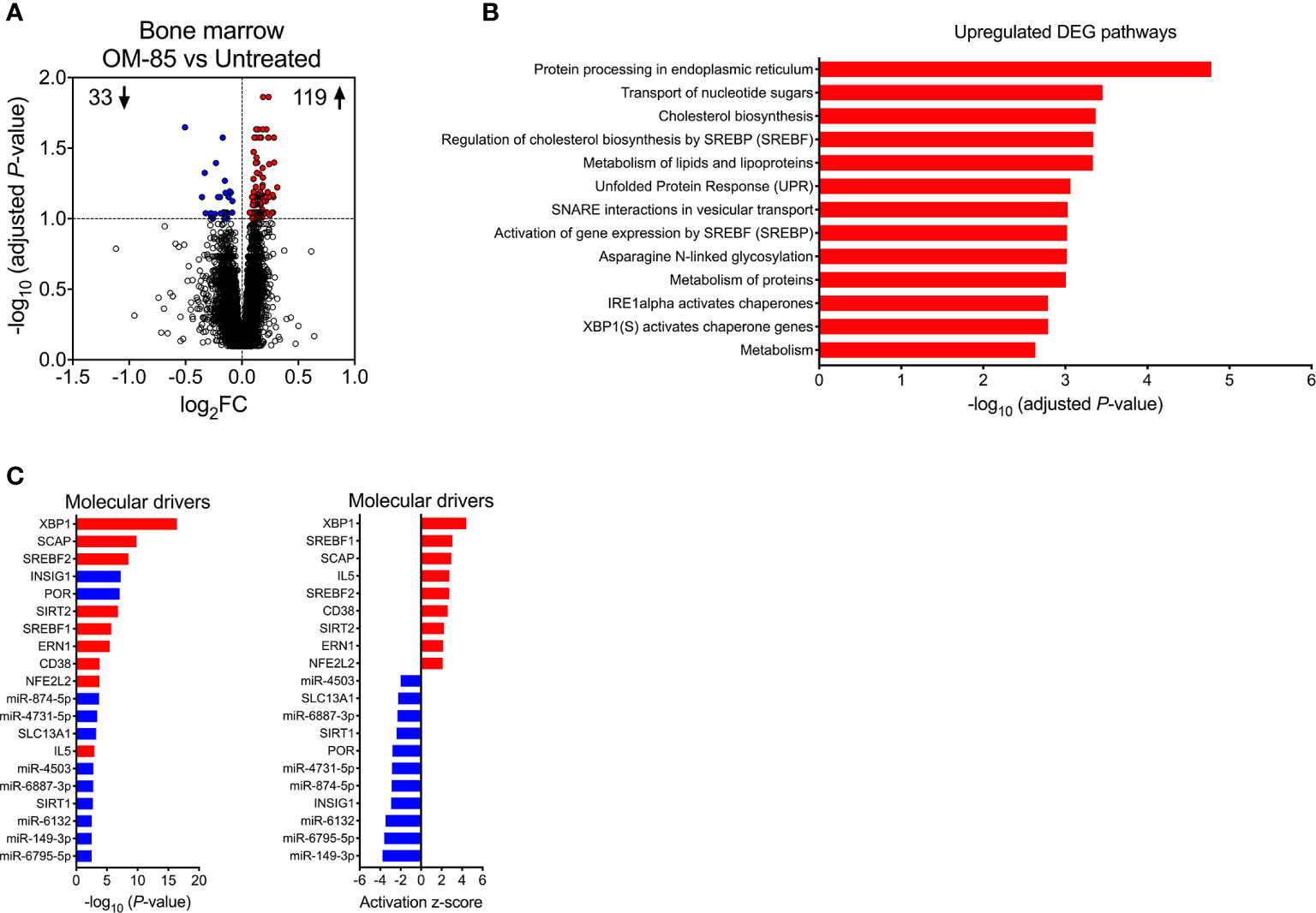
Figure 3 Maternal OM-85-induced changes in fetal bone marrow gene expression profiles. (A) DEG within fBM comparing fetuses from OM-85-treated and untreated mothers. DEG are summarized as a volcano plot showing data along axes of statistical significance (-log10 adjusted P-value) and differential expression magnitude (log2 fold change) for n = 16 individual animals per group collected from n = 7 independent experiments. Dashed horizontal lines indicate a False Discovery Rate (FDR) adjusted P-value < 0.1. Genes shown in red were upregulated and those shown in blue were downregulated. (B) Top biological pathways associated with upregulated DEG within fBM comparing fetuses from OM-85-treated and untreated mothers. (C) Activated (red) and inhibited (blue) molecular drivers of the differential expression patterns were identified using Upstream Regulator Analysis.
Upstream regulator analysis was then performed to identify putative molecular drivers of all observed DEG. The data revealed X-box binding protein 1 (XBP1), a transcription factor central to the UPR (38) and crucial in the development, survival and function of multiple cell types including plasma cells (39), eosinophils (40), natural killer (NK) cells (41), T-cell subsets (42) and DCs (43, 44), as the most strongly activated molecular driver within fBM associated with maternal OM-85 treatment effects (P-value = 3.81x10-17, Z-score = 4.427, Figure 3C; Supplementary Table 3), and consistent with this, its downstream target, the canonical UPR sensor Activating Transcription Factor 6 beta (ATF6b) (45, 46) was upregulated (Supplementary Table 1 and Supplementary Table 3). Additionally, Endoplasmic Reticulum To Nucleus Signalling 1 (ERN1) was identified as an activated driver gene within the fBM following maternal OM-85 treatment (P-value = 3.32x10-6, Z-score = 2.156; Figure 3C; Supplementary Table 3). Identification of ERN1 is crucial given that during the ER stress response, this gene encodes the ER stress sensor protein inositol-requiring enzyme 1 (IRE1α), responsible for the unconventional cleavage of a 26 nucleotide fragment from Xbp1 mRNA, resulting in the generation of the active spliced variant of XBP1 (XBP1s) and enabling it to function as a potent transcription factor within the UPR signalling pathway (46), as evidenced by XBP1 being identified as a downstream target gene of activated ERN1 (Supplementary Table 3). Collectively, these findings suggest that activation of the UPR pathway may be a central component of the immune training mechanism induced in fBM as a result of maternal OM-85 treatment. In addition to UPR pathway drivers, maternal OM-85 treatment also resulted in the upstream activation of multiple drivers central to immunometabolic pathways involved in cellular cholesterol homeostasis, including Sterol Regulatory Element Binding Transcription Factor 1 (SREBF1; P-value = 1.95x10-6, Z-score = 3.056) and 2 (SREBF2; P-value = 2.95x10-9, Z-score = 2.745) and Sterol Regulatory Element Binding Protein Cleavage-Activating Protein (SCAP; P-value = 1.52x10-10, Z-score = 2.949; Figure 3C; Supplementary Table 3). This was associated with upregulation of their downstream target Low-Density Lipoprotein Receptor (LDLR; Supplementary Table 1 and Supplementary Table 3), while Insulin Inducible Gene 1 (INSIG1) was inhibited in fBM following maternal OM-85 treatment (P-value = 5.81x10-8, Z-score = −2.931; Figure 3C; Supplementary Table 3).
Additional candidate drivers in fBM following maternal OM-85 treatment included CD38, IL5, and an array of microRNAs (miR) (Figure 3C; Supplementary Table 3) recognized principally in the context of cancer-associated functions (47–49). Of note, miR-149-3p (strongly downregulated in Figure 3C) has been shown to negatively regulate Toll-like receptor (TLR) 4 expression in murine monocytic cells in vitro (50) and it is possible that other miRs may have similar (but as yet undefined) innate immune regulatory functions (51). The relevance of finding TLR4 upregulation in BM-derived myeloid cells in this model merits further investigation. Likewise, the identification of the T-cell activation-associated markers CD38 and IL-5 suggests possible contributions from activated T-cells, and these possibilities will also be addressed in follow up studies.
Upregulation of XBP1s Expression Is Restricted to Fetal Bone Marrow cDC Precursors
Finally, to obtain evidence confirming that the activated form of XBP1 was upregulated, we measured expression of the active spliced variant of XBP1 (XBP1s) at the protein level within fBM. Using multicolor flow cytometry (Supplementary Figure 5), we identified significant upregulation of fBM CD11b+CD11c+ pre-cDCs expressing intracellular XBP1s following maternal OM-85 treatment (Figures 4A, B). While intracellular XBP1s expression was additionally localized within fetal B-cells, NK cells and T-cells (Supplementary Figure 6), as previously described in the literature (39, 41, 42, 52), maternal OM-85 treatment had no impact on XBP1s expression levels in these cell types.
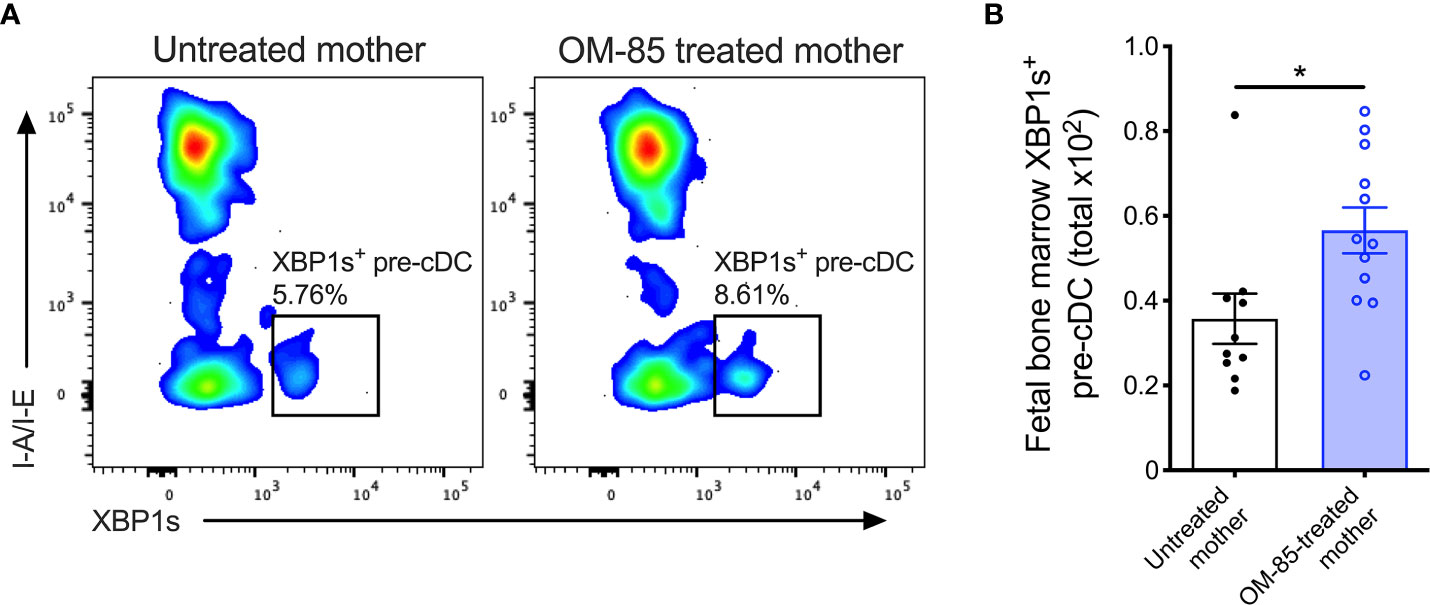
Figure 4 XBP1s expression in pre-cDCs within fetal bone marrow. (A) Representative flow cytometry plots demonstrating intracellular XBP1s expression in CD11b+B220-CD11c+Gr-1-I-A/I-E-XBP1s+ pre-cDC within fBM. (B) Absolute numbers of XBP1s+ pre-cDC in BM of fetuses from OM-85-treated and untreated mothers. Data are presented from individual animals comparing fetuses from OM-85-treated and untreated mothers and displayed as bar graphs showing mean ± SEM of n = 4 independent experiments, with each experiment containing fetuses from 1 untreated and/or 1 OM-85 treated mother. Statistical significance was determined using Mann-Whitney U test. *p < 0.05.
Discussion
In this study, we have characterized the response of the fBM myeloid cell compartment to maternal treatment with the microbial-derived innate immune modulator OM-85 during pregnancy. We demonstrate that, consistent with our previous reports in 6-week-old offspring (15), maternal OM-85 treatment expanded the baseline pool of GMP and MDP in fBM. Furthermore, we show for the first time the maternal OM-85-induced modulation of metabolic pathways within fBM responsible for cellular cholesterol homeostasis, with specific activation of SREBF1, SREBF2, SCAP and LDLR. In this regard, SREBF1 and SREBF2 are responsible for encoding sterol regulatory element binding proteins (SREBP) which play a central role in cellular metabolism by controlling the synthesis of cholesterol and other membrane lipids in the Golgi of mammalian cells, with SREBP2 (encoded by SREBF2) regarded as the “master regulator” of cellular cholesterol biosynthesis (53). Furthermore, SCAP is central to this process by acting as a protein chaperone, mediating the transport of SREBP from the ER to the Golgi where it can promote transcription of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme of the mevalonate pathway responsible for intracellular cholesterol biosynthesis (53). In parallel with this metabolic pathway, LDLR regulates cellular uptake of low-density lipoprotein (LDL), the upregulation of which increases downstream cholesterol accumulation within the cell and is required for the proliferation of hematopoietic stem and progenitor cells (54). It is now recognized that myelopoiesis within the BM is heavily reliant upon an increased demand for cellular cholesterol and enhanced cholesterol biosynthesis (55–57), and we therefore postulate that upregulation of this immunometabolic pathway following maternal OM-85 treatment is in-part responsible for the expansion of MP, GMP and MDP observed within fBM. Further reinforcing the importance of enhanced cholesterol biosynthesis, INSIG1, an ER membrane protein that prevents trafficking of SCAP/SREBP complexes to the Golgi and thereby terminates cholesterol biosynthesis by restricting HMG-CoA reductase-mediated activation of the mevalonate pathway (53, 58), was inhibited in fBM following maternal OM-85 treatment. Together, these findings parallel previous studies demonstrating that activation of cellular cholesterol biosynthesis and resultant expansion of BM MP is a hallmark of classical β-glucan-mediated central immune training, and suggests a common role for the mevalonate pathway in these immune training mechanisms (10, 59). We further demonstrate that transplacental mechanisms promoting maternal OM-85-induced immune training within fBM involve a dynamic process comprising upregulation of immunometabolic pathways that provide key rate-limiting metabolites required for myelopoiesis and subsequent expansion of MP, GMP and MDP, and associated inhibition of negative feedback loops responsible for arresting cholesterol biosynthesis.
Downstream of the fBM progenitor response, maternal OM-85 treatment selectively amplified the overall abundance of fBM cDC, along with enhancing the concomitant functional maturation of these cDC as demonstrated by upregulated I-A/I-E (MHC Class II) expression. This BM population is the source of the precursors which subsequently seed the airway mucosal DC network that progressively develops between birth and weaning (3, 4). It is noteworthy that the cDC which initially seed this network postnatally are MHC-IIlow (reflecting their functionally immature status) relative to the high-level expression seen at later ages (3), and the findings above in fetal cDC from the treated group may collectively explain the accelerated postnatal establishment and the enhanced functional maturation of this network observed in their offspring (15). This DC network plays an essential “gatekeeper” role in immune surveillance of airway surfaces, and hence in protection against both allergic and infectious diseases in the respiratory tract (60, 61), and its relative paucity and reduced functionality during infancy may be an important contributor to increased susceptibility to these diseases during this life phase.
Earlier studies from our group also identified unique features of the population dynamics of this lung cDC network which distinguishes it from comparable populations in other tissues, notably the exceptionally rapid baseline turnover rate of individual cells within the network, ~85% of which are replaced every 24–36 h (62), with emigration to draining lymph nodes (bearing samples of locally acquired antigens) balanced by recruitment of replacements from BM. Moreover, once development of functional competence is complete (4), this network develops capability for rapid expansion to up to 5-fold baseline density in the face of acute challenge with airborne pro-inflammatory irritant, allergenic or microbial stimuli (63), the latter response exhibiting kinetics that rival neutrophils (64, 65). These unique population dynamics suggest that even at baseline, mechanisms that promote lung cDC survival are likely to play a crucial role in the capacity of the network to perform its immune surveillance functions which require onward migration to downstream lymph nodes and subsequent interaction with T-cells as opposed to antigen presentation in situ (60); moreover during prolonged/severe events exemplified by severe viral infections, the added effects of cDC injury resulting from inflammation-associated ER stress (66) would place further pressure on survival times.
In this regard, our transcriptomic analyses of fBM from offspring of OM-85-treated mothers also identified upregulated expression of XBP1, ATF6β and ERN1 (encoding IRE1α), key components of the XBP1-ERN1 signalling axis and critical regulators of the UPR pathway (67) which mitigates the effects of ER stress, and moreover we localized upregulated production of active XBP1s protein to cDC precursors. While this enhanced XBP1s+ cDC precursor pool constitutes a small population within the totality of the fBM, individual precursor cells have a remarkable ability to generate vast pools of mature DC (68), and as such heavily influence the downstream function of peripheral tissue DC populations. Taken together with recent findings on the role for IRE1α-XBP1 signalling and the downstream transcription factor XBP1s in DC development and function (43, 44), these results suggest a central role for the XBP1-ERN signalling axis in this OM-85-mediated immune training process.
In further support of this suggestion, other studies demonstrate a significant reduction in CD11c+ cells (mirroring that of our pre-cDC phenotype) within XBP1-/- BM cultures, whilst forced overexpression of XBP1s in XBP1-/- DC precursors conversely rescues and subsequently drives expansion of the DC pool in vitro (43). It is also pertinent to note that others have reported that the cDC population in the lung mucosa is differentially reliant upon XBP1 expression for survival at baseline relative to cDC from other tissue sites (66), which may be a direct reflection of the uniquely high turnover rates of cDC within the airway mucosal microenvironment (62).
Collectively, the data presented here indicate that OM-85 likely operates as an immune training agent, employing cellular and immunometabolic mechanisms previously reported in independent model systems (10, 11, 69), with the additional capacity to act transplacentally via the fBM. Furthermore, we go beyond the currently known features of innate immune training to describe involvement of the XBP1-ERN1 signalling axis. Moreover, classical β-glucan- and BCG-mediated immune training has traditionally focused on prototypic innate effector cell populations (monocytes/macrophages/natural killer cells) resulting in enhanced resistance to bystander pathogens via the upregulation of pro-inflammatory responses exemplified by tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), IL-6 and IL-1β production (70–72) and emergency granulopoiesis-mediated neutrophilic influx (69). However, studies are now beginning to recognize that immune training can also occur in DC populations, resulting inter alia in epigenetic reprograming of pro-inflammatory cytokine responses (73). We have recently extended these observations to include OM-85-mediated training effects on key immunoregulatory functions in both pregnant mice and their offspring, including effects on both cDC and pDC populations and downstream T-regulatory cells, which are collectively associated with enhanced resistance of both mothers and offspring to the pro-inflammatory effects of bacterial, viral and allergenic stimulation (15, 18). Of note, similar transplacental immune training-like effects targeting immunoregulatory mechanisms in offspring have been reported in relation to pregnant maternal exposure to extracts from Acinetobacter lwoffii (74) and Helicobacter pylori (75), suggesting that the phenomenon reported here may be generalizable, and may point towards a novel approach to mitigation of disease risk in the age group that is in greatest need of protection.
Data Availability Statement
The dataset generated for this study can be found in the Gene Expression Omnibus repository: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE140143.
Ethics Statement
The animal study was reviewed and approved by Telethon Kids Institute Animal Ethics Committee.
Author Contributions
KM, PH, and DS designed the study. KM, MB, NS, and J-FL-J performed the experiments. KM, AJ, MB, and DS analyzed the data. PS and AB contributed to the project design and discussions on data interpretation. KM, PS, PH, and DS wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Health and Medical Research Council of Australia (APP1047212).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to acknowledge the animal technicians at the Telethon Kids Institute Bioresources Centre. This manuscript has been released as a pre-print at bioRxiv (76).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.601494/full#supplementary-material
References
1. Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol (2007) 7(5):379–90. doi: 10.1038/nri2075
2. Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol (2004) 4(7):553–64. doi: 10.1038/nri1394
3. Nelson DJ, McMenamin C, McWilliam AS, Brenan M, Holt PG. Development of the airway intraepithelial dendritic cell network in the rat from class II major histocompatibility (Ia)-negative precursors: differential regulation of Ia expression at different levels of the respiratory tract. J Exp Med (1994) 179(1):203–12. doi: 10.1084/jem.179.1.203
4. Nelson DJ, Holt PG. Defective regional immunity in the respiratory tract of neonates is attributable to hyporesponsiveness of local dendritic cells to activation signals. J Immunol (1995) 155(7):3517–24.
5. Gollwitzer ES, Marsland BJ. Impact of Early-Life Exposures on Immune Maturation and Susceptibility to Disease. Trends Immunol (2015) 36(11):684–96. doi: 10.1016/j.it.2015.09.009
6. Apostol AC, Jensen KDC, Beaudin AE. Training the Fetal Immune System Through Maternal Inflammation—A Layered Hygiene Hypothesis. Front Immunol (2020) 11(123):1–14. doi: 10.3389/fimmu.2020.00123
7. von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol (2010) 10(12):861–8. doi: 10.1038/nri2871
8. Ober C, Sperling AI, von Mutius E, Vercelli D. Immune development and environment: lessons from Amish and Hutterite children. Curr Opin Immunol (2017) 48:51–60. doi: 10.1016/j.coi.2017.08.003
9. Kleinnijenhuis J, Quintin J, Preijers F, Benn CS, Joosten LA, Jacobs C, et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun (2014) 6(2):152–8. doi: 10.1159/000355628
10. Mitroulis I, Ruppova K, Wang B, Chen LS, Grzybek M, Grinenko T, et al. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell (2018) 172(1-2):147–61. doi: 10.1016/j.cell.2017.11.034
11. Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonça LE, Pacis A, et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell (2018) 172(1):176–90.e19. doi: 10.1016/j.cell.2017.12.031
12. Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol (2020) 20:375–88. doi: 10.1038/s41577-020-0285-6
13. Mulder WJM, Ochando J, Joosten LAB, Fayad ZA, Netea MG. Therapeutic targeting of trained immunity. Nat Rev Drug Discov (2019) 18(7):553–66. doi: 10.1038/s41573-019-0025-4
14. Holt PG, Strickland DH, Custovic A. Targeting maternal immune function during pregnancy for asthma prevention in offspring: Harnessing the “farm effect”? J Allergy Clin Immunol (2020) 146(2):270–2. doi: 10.1016/j.jaci.2020.04.008
15. Mincham KT, Scott NM, Lauzon-Joset JF, Leffler J, Larcombe AN, Stumbles PA, et al. Transplacental immune modulation with a bacterial-derived agent protects against allergic airway inflammation. J Clin Invest (2018) 128(11):4856–69. doi: 10.1172/jci122631
16. Razi CH, Harmanci K, Abaci A, Özdemir O, Hizli Ş, Renda R, et al. The immunostimulant OM-85 BV prevents wheezing attacks in preschool children. J Allergy Clin Immunol (2010) 126(4):763–9. doi: 10.1016/j.jaci.2010.07.038
17. Rozy A, Chorostowska-Wynimko J. Bacterial immunostimulants–mechanism of action and clinical application in respiratory diseases. Pneumonol Alergol Pol (2008) 76(5):353–9.
18. Scott NM, Lauzon-Joset JF, Jones AC, Mincham KT, Troy NM, Leffler J, et al. Protection against maternal infection-associated fetal growth restriction: proof-of-concept with a microbial-derived immunomodulator. Mucosal Immunol (2017) 10(3):789–801. doi: 10.1038/mi.2016.85
19. Strickland DH, Judd S, Thomas JA, Larcombe AN, Sly PD, Holt PG. Boosting airway T-regulatory cells by gastrointestinal stimulation as a strategy for asthma control. Mucosal Immunol (2011) 4(1):43–52. doi: 10.1038/mi.2010.43
20. Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika (1986) 73(1):13–22. doi: 10.1093/biomet/73.1.13
21. de Souza W, Carvalho BdS, Lopes-Cendes I. Rqc: A Bioconductor Package for Quality Control of High-Throughput Sequencing Data. J Stat Software Code Snippets (2018) 87(2):1–14. doi: 10.18637/jss.v087.c02
22. Liao Y, Smyth GK, Shi W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res (2013) 41(10):e108. doi: 10.1093/nar/gkt214
23. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol (2014) 15(12):550. doi: 10.1186/s13059-014-0550-8
24. Breuer K, Foroushani AK, Laird MR, Chen C, Sribnaia A, Lo R, et al. InnateDB: systems biology of innate immunity and beyond-recent updates and continuing curation. Nucleic Acids Res (2013) 41(Database issue):D1228–33. doi: 10.1093/nar/gks1147
25. Krämer A, Green J, Pollard J Jr., Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics (2014) 30(4):523–30. doi: 10.1093/bioinformatics/btt703
26. Moorlag SJCFM, Khan N, Novakovic B, Kaufmann E, Jansen T, van Crevel R, et al. β-Glucan Induces Protective Trained Immunity against Mycobacterium tuberculosis Infection: A Key Role for IL-1. Cell Rep (2020) 31(7):107634. doi: 10.1016/j.celrep.2020.107634
27. Yáñez A, Coetzee SG, Olsson A, Muench DE, Berman BP, Hazelett DJ, et al. Granulocyte-Monocyte Progenitors and Monocyte-Dendritic Cell Progenitors Independently Produce Functionally Distinct Monocytes. Immunity (2017) 47(5):890–902.e4. doi: 10.1016/j.immuni.2017.10.021
28. Wolf AA, Yáñez A, Barman PK, Goodridge HS. The Ontogeny of Monocyte Subsets. Front Immunol (2019) 10(1642):1–8. doi: 10.3389/fimmu.2019.01642
29. Drissen R, Buza-Vidas N, Woll P, Thongjuea S, Gambardella A, Giustacchini A, et al. Distinct myeloid progenitor-differentiation pathways identified through single-cell RNA sequencing. Nat Immunol (2016) 17(6):666–76. doi: 10.1038/ni.3412
30. Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature (2000) 404(6774):193–7. doi: 10.1038/35004599
31. Iwasaki H, Mizuno S-I, Mayfield R, Shigematsu H, Arinobu Y, Seed B, et al. Identification of eosinophil lineage–committed progenitors in the murine bone marrow. J Exp Med (2005) 201(12):1891–7. doi: 10.1084/jem.20050548
32. Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science (2006) 311(5757):83–7. doi: 10.1126/science.1117729
33. Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, et al. CX(3)CR1(+) CD115(+) CD135(+) common macrophage/DC precursors and the role of CX(3)CR1 in their response to inflammation. J Exp Med (2009) 206(3):595–606. doi: 10.1084/jem.20081385
34. Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell (1997) 91(5):661–72. doi: 10.1016/S0092-8674(00)80453-5
35. Karsunky H, Inlay MA, Serwold T, Bhattacharya D, Weissman IL. Flk2+ common lymphoid progenitors possess equivalent differentiation potential for the B and T lineages. Blood (2008) 111(12):5562–70. doi: 10.1182/blood-2007-11-126219
36. Carotta S, Pang SHM, Nutt SL, Belz GT. Identification of the earliest NK-cell precursor in the mouse BM. Blood (2011) 117(20):5449–52. doi: 10.1182/blood-2010-11-318956
37. Fathman JW, Bhattacharya D, Inlay MA, Seita J, Karsunky H, Weissman IL. Identification of the earliest natural killer cell-committed progenitor in murine bone marrow. Blood (2011) 118(20):5439–47. doi: 10.1182/blood-2011-04-348912
38. Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol (2003) 23(21):7448–59. doi: 10.1128/MCB.23.21.7448-7459.2003
39. Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature (2001) 412(6844):300–7. doi: 10.1038/35085509
40. Bettigole SE, Lis R, Adoro S, Lee AH, Spencer LA, Weller PF, et al. The transcription factor XBP1 is selectively required for eosinophil differentiation. Nat Immunol (2015) 16(8):829–37. doi: 10.1038/ni.3225
41. Dong H, Adams NM, Xu Y, Cao J, Allan DSJ, Carlyle JR, et al. The IRE1 endoplasmic reticulum stress sensor activates natural killer cell immunity in part by regulating c-Myc. Nat Immunol (2019) 20(7):865–78. doi: 10.1038/s41590-019-0388-z
42. Pramanik J, Chen X, Kar G, Henriksson J, Gomes T, Park JE, et al. Genome-wide analyses reveal the IRE1a-XBP1 pathway promotes T helper cell differentiation by resolving secretory stress and accelerating proliferation. Genome Med (2018) 10(1):76. doi: 10.1186/s13073-018-0589-3
43. Iwakoshi NN, Pypaert M, Glimcher LH. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J Exp Med (2007) 204(10):2267–75. doi: 10.1084/jem.20070525
44. Osorio F, Tavernier SJ, Hoffmann E, Saeys Y, Martens L, Vetters J, et al. The unfolded-protein-response sensor IRE-1alpha regulates the function of CD8alpha+ dendritic cells. Nat Immunol (2014) 15(3):248–57. doi: 10.1038/ni.2808
45. Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, et al. Endoplasmic reticulum stress-induced formation of transcription factor complex ERSF including NF-Y (CBF) and activating transcription factors 6alpha and 6beta that activates the mammalian unfolded protein response. Mol Cell Biol (2001) 21(4):1239–48. doi: 10.1128/MCB.21.4.1239-1248.2001
46. Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell (2001) 107(7):881–91. doi: 10.1016/S0092-8674(01)00611-0
47. Xia B, Lin M, Dong W, Chen H, Li B, Zhang X, et al. Upregulation of miR-874-3p and miR-874-5p inhibits epithelial ovarian cancer malignancy via SIK2. J Biochem Mol Toxicol (2018) 32(8):e22168. doi: 10.1002/jbt.22168
48. Stark MS, Tom LN, Boyle GM, Bonazzi VF, Soyer HP, Herington AC, et al. The “melanoma-enriched” microRNA miR-4731-5p acts as a tumour suppressor. Oncotarget (2016) 7(31):49677–87. doi: 10.18632/oncotarget.10109
49. Yang D, Du G, Xu A, Xi X, Li D. Expression of miR-149-3p inhibits proliferation, migration, and invasion of bladder cancer by targeting S100A4. Am J Cancer Res (2017) 7(11):2209–19.
50. Shen W, Liu J, Zhao G, Fan M, Song G, Zhang Y, et al. Repression of Toll-like receptor-4 by microRNA-149-3p is associated with smoking-related COPD. Int J Chron Obstruct Pulmon Dis (2017) 12:705–15. doi: 10.2147/copd.S128031
51. Kirchner B, Pfaffl MW, Dumpler J, von Mutius E, Ege MJ. microRNA in native and processed cow’s milk and its implication for the farm milk effect on asthma. J Allergy Clin Immunol (2016) 137(6):1893–5.e13. doi: 10.1016/j.jaci.2015.10.028
52. Todd DJ, McHeyzer-Williams LJ, Kowal C, Lee AH, Volpe BT, Diamond B, et al. XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. J Exp Med (2009) 206(10):2151–9. doi: 10.1084/jem.20090738
53. Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell (2006) 124(1):35–46. doi: 10.1016/j.cell.2005.12.022
54. Tolani S, Pagler TA, Murphy AJ, Bochem AE, Abramowicz S, Welch C, et al. Hypercholesterolemia and reduced HDL-C promote hematopoietic stem cell proliferation and monocytosis: studies in mice and FH children. Atherosclerosis (2013) 229(1):79–85. doi: 10.1016/j.atherosclerosis.2013.03.031
55. Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science (New York NY) (2010) 328(5986):1689–93. doi: 10.1126/science.1189731
56. Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo C-L, et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest (2011) 121(10):4138–49. doi: 10.1172/JCI57559
57. Morgan PK, Fang L, Lancaster GI, Murphy AJ. Hematopoiesis is regulated by cholesterol efflux pathways and lipid rafts: connections with cardiovascular diseases. J Lipid Res (2020) 61(5):667–75. doi: 10.1194/jlr.TR119000267
58. Yang T, Espenshade PJ, Wright ME, Yabe D, Gong Y, Aebersold R, et al. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell (2002) 110(4):489–500. doi: 10.1016/s0092-8674(02)00872-3
59. Bekkering S, Arts RJW, Novakovic B, Kourtzelis I, van der Heijden CDCC, Li Y, et al. Metabolic Induction of Trained Immunity through the Mevalonate Pathway. Cell (2018) 172(1):135–46.e9. doi: 10.1016/j.cell.2017.11.025
60. Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol (2008) 8(2):142–52. doi: 10.1038/nri2236
61. Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol (2012) 30:243–70. doi: 10.1146/annurev-immunol-020711-075021
62. Holt PG, Haining S, Nelson DJ, Sedgwick JD. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J Immunol (1994) 153(1):256–61.
63. McWilliam AS, Napoli S, Marsh AM, Pemper FL, Nelson DJ, Pimm CL, et al. Dendritic Cells Are Recruited into the Airway Epithelium during the Inflammatory Response to a Broad Spectrum of Stimuli. J Exp Med (1996) 184(6):2429–32. doi: 10.1084/jem.184.6.2429
64. Jahnsen FL, Strickland DH, Thomas JA, Tobagus IT, Napoli S, Zosky GR, et al. Accelerated antigen sampling and transport by airway mucosal dendritic cells following inhalation of a bacterial stimulus. J Immunol (2006) 177(9):5861–7. doi: 10.4049/jimmunol.177.9.5861
65. McWilliam AS, Nelson D, Thomas JA, Holt PG. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med (1994) 179(4):1331–6. doi: 10.1084/jem.179.4.1331
66. Tavernier SJ, Osorio F, Vandersarren L, Vetters J, Vanlangenakker N, Van Isterdael G, et al. Regulated IRE1-dependent mRNA decay sets the threshold for dendritic cell survival. Nat Cell Biol (2017) 19(6):698–710. doi: 10.1038/ncb3518
67. Grootjans J, Kaser A, Kaufman RJ, Blumberg RS. The unfolded protein response in immunity and inflammation. Nat Rev Immunol (2016) 16(8):469–84. doi: 10.1038/nri.2016.62
68. Naik SH, Sathe P, Park H-Y, Metcalf D, Proietto AI, Dakic A, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol (2007) 8(11):1217–26. doi: 10.1038/ni1522
69. Brook B, Harbeson DJ, Shannon CP, Cai B, He D, Ben-Othman R, et al. BCG vaccination–induced emergency granulopoiesis provides rapid protection from neonatal sepsis. Sci Transl Med (2020) 12(542):eaax4517. doi: 10.1126/scitranslmed.aax4517
70. Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe (2012) 12(2):223–32. doi: 10.1016/j.chom.2012.06.006
71. Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A (2012) 109(43):17537–42. doi: 10.1073/pnas.1202870109
72. Sun JC, Beilke JN, Lanier LL. Adaptive Immune Features of Natural Killer Cells. Nature (2009) 457(7229):557–61. doi: 10.1038/nature07665
73. Hole CR, Wager CML, Castro-Lopez N, Campuzano A, Cai H, Wozniak KL, et al. Induction of memory-like dendritic cell responses in vivo. Nat Commun (2019) 10(1):2955. doi: 10.1038/s41467-019-10486-5
74. Conrad ML, Ferstl R, Teich R, Brand S, Blumer N, Yildirim AO, et al. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J Exp Med (2009) 206(13):2869–77. doi: 10.1084/jem.20090845
75. Kyburz A, Fallegger A, Zhang X, Altobelli A, Artola-Boran M, Borbet T, et al. Transmaternal Helicobacter pylori exposure reduces allergic airway inflammation in offspring through regulatory T cells. J Allergy Clin Immunol (2019) 143(4):1496–512.e11. doi: 10.1016/j.jaci.2018.07.046
Keywords: innate immune training, myelopoiesis, immunomodulator, XBP1, dendritic cell, transplacental, myeloid progenitor, OM-85
Citation: Mincham KT, Jones AC, Bodinier M, Scott NM, Lauzon-Joset J-F, Stumbles PA, Bosco A, Holt PG and Strickland DH (2020) Transplacental Innate Immune Training via Maternal Microbial Exposure: Role of XBP1-ERN1 Axis in Dendritic Cell Precursor Programming. Front. Immunol. 11:601494. doi: 10.3389/fimmu.2020.601494
Received: 01 September 2020; Accepted: 05 November 2020;
Published: 02 December 2020.
Edited by:
Chien-Kuo Lee, National Taiwan University, TaiwanReviewed by:
Yves Laumonnier, University of Lübeck, GermanyHarissios Vliagoftis, University of Alberta, Canada
Inken Schmudde, University of Lübeck, Germany
Copyright © 2020 Mincham, Jones, Bodinier, Scott, Lauzon-Joset, Stumbles, Bosco, Holt and Strickland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deborah H. Strickland, Deb.Strickland@telethonkids.org.au
†These authors share senior authorship
 Kyle T. Mincham
Kyle T. Mincham Anya C. Jones
Anya C. Jones Marie Bodinier
Marie Bodinier Naomi M. Scott1
Naomi M. Scott1 Jean-Francois Lauzon-Joset
Jean-Francois Lauzon-Joset Philip A. Stumbles
Philip A. Stumbles Anthony Bosco
Anthony Bosco Deborah H. Strickland
Deborah H. Strickland