Abstract
The high enhancement factor (EF) of surface-enhanced Raman (SERS) probes is an important parameter for high-sensitivity Raman scattering measurement applied to environment control. In this article, we present a SERS probe based on silver nano-structures deposited on silica microsphere surfaces made by the laser-assisted photochemical method and reflection converging mirror for a high EF Raman measurement of ultralow organic compound concentration inliquid environments. The laser-assisted photochemical method can synthesize and homogeneously deposit nano-silvers on a silica microsphere, and the reflection converging mirror can collect more Raman signal to the photo-detector and give high Raman enhancement of the SERS probe. The SERS-activity of the probe is verified by the detection of ultralow concentrations of Rhodamine 6 G in aqueous solutions in the range of 10−4–10−10 M. The obtained results show that the proposed SERS probe has an increase of collected Raman intensity up to 32% in comparison to a SERS probe without reflection converging mirror, and an EF of the SERS probe achieved up to 1.8 × 1010. We believe that the novel SERS probe has a large potential for applications in biochemical sensing techniques.
Export citation and abstract BibTeX RIS
1. Introduction
In recent years, photonic sensing technology has been rapidly developed for applications in environmental monitoring, control of food safety and health care [1–4]. Among the photonic technologies applied to biochemical sensing applications, surface-enhanced Raman (SERS) has been shown to have high sensitivity and selective detections for biochemical molecules [5–7]. The Raman signal enhancement of SERS strongly depends on a highly local, enhanced electromagnetic (EM) field on the nano-metallic surface due to the excitation of localized surface plasmon resonances (LSPR) and on the enhanced chemical interaction between the adsorbate and noble metal nanoparticles [8–11]. Recently, there have been several investigations combining nano-metallic structures with optical fibres for SERS activity [12–17]. Biochemical fibre sensors are a rapidly developing area because of their advantages such as low optical attenuation, small sensing active area, free optical adjustment, immunity from electromagnetic field interference, and their being well-adapted to portable equipment. From noble metals such as gold, silver, palladium, and platinum that have normally been applied in SERS substrates, silver nanostructures are considered as excellent candidates for SERS for their high-LSPR properties, easily fabricating different morphologies such as quantum dots, nanodiscs, flower-like morphologies, and nanodendrites. SERS substrates based on Ag-nanostructures can be fabricated in many different ways with various morphologies, such as gamma irradiation, electron irradiation, electrochemical deposition, chemical vapour deposition, chemical synthesis, microwave processing, hydrothermal and photochemical methods [18–22]. For the detection of ultralow concentrations of testing materials, a high enhancement factor (EF) and measuring repetition of the SERS probe are important features. As we have known, the Raman signal enhancement highly depends on the arrangement, distribution, and morphology of the nano-metal surface. Furthermore, a collection of Raman signals is commonly carried out from a single side of the SERS substrate that cannot collect the total Raman signal intensity, because the Raman scattering emission would be radiated around the nano-metal structures.
In this article, we propose a SERS probe using silver (Ag)-nanostructures deposited on a silica glass microsphere combined with a reflection converging mirror for increasing the collected Raman scattering signal from the silica microsphere surface. The Ag-nano structures deposited on the silica microsphere have been fabricated by laser-assisted photochemical technique, which will take a one-time synthesis and deposition of Ag-nanostructures on the silica microsphere surface in comparison with other chemical methods. The morphology and chemical composition of SERS probes are characterized by a high-resolution scanning electron microscope (HR-SEM) and energy dispersive x-ray spectroscopy (EDX), respectively. The SERS-activity of the nano-Ag-coated silica microsphere probes are demonstrated with detections of ultralow concentrations of Rhodamine 6 G in aqueous solutions in the range of 10−4–10−10 M. The obtained Raman signal of the proposed SERS probe based on Ag-nanostructures on the microsphere surface and reflection converging mirrors increased up to 32% in comparison with non-reflection SERS, and the (EF of the novel SERS probe achieved up to 1010 M for detecting Rhodamine 6 G at the 10−10 M concentration in aqueous solutions. This SERS probe is a promising candidate for detection of biochemical compounds with ultralow concentrations in the liquid environment.
2. Experimental section
A laser-assisted photochemical method for making Ag-nano dendrites deposited on silica fibre flatends for SERS substrates was previously developed by our group [23]. The development of SERS based on nano-Ag coated on the silica microsphere has a purpose of obtaining the homogenous distribution of nano-Ag on the substrate. The making process for Ag-nano structures deposited on the silica microsphere is carried out by two main steps: (i) the first step is to make a silica microsphere from etched optical fibre by an electric arc; and (ii) the second is to prepare Ag-nanostructures on the microsphere surface from Ag-ion consisted solution by a laser-assisted photochemical method using green laser irradiation through an optical fibre. A schematic diagram of the making process of SERS probes with Ag nanostructures on silica microspheres, prepared in two steps, is shown in figure 1. A silica microsphere probe on multimode optical fibre with core/cladding diameters of 50/125 microns, supplied from Thorlabs (USA), was fabricated by wet chemical etching the end of the optical fibre in 30%- HF acid solution for 30 min and then forming a microsphere by melting the etched fibre end using an electrical arc between a couple of discharge electrodes, as shown in figure 1(a). The Ag-ion solution was prepared by mixing an optimal molar concentration of aqueous AgNO3 with 0.1 mM (Fisher Scientific, UK), and C6H5Na3O7.2H2O of 3 mM (Merck KGaA, Germany) in a volume ratio of 1:1 with rapid stirring for 5 min, then a freshly prepared 0.02 mM NaBH4 solution (Kanto Chemical, Japan) was added by drop wise addition to the mixture under strong stirring for 15 min, and, after that the obtained product was kept in the dark at room temperature. The growth and deposition of Ag nanostructures on surface microspheres were performed by a green laser with an emission wavelength of 532 nm (Laserlands, China), which propagated through the optical fibre to the silica microsphere attached at the end of this fibre, as shown in figure 1(b). The green laser beam was transmitted into the fibre via a grin-rod lens and FC/PC standard connector, similarly to the scheme presented in [23], so the laser beam effectively coupled to the microsphere-attached optical fibre. The microsphere-attached fibre was dipped into the prepared Ag-ion solution, and then the green laser beam with an optical power of about 80 W cm−2 was led into the solution via this optical fibre. Growth and deposition of Ag nanostructures can be one-time formed on the light-focused area of the microsphere surface after illumination of a few minutes. In our experiment, the Ag-growth solution was contained in a box with temperatures at 3 °C–5 °C stabilized by a Peltier thermoelectric cooler to avoid the influence of thermal effect on the growth process of Ag nanostructure [24]. After the silver growth and deposition process, the microsphere probe was carefully rinsed in deionized water and dried with a pure nitrogen gas stream.
Figure 1. Experimental setup for manufacturing Ag-nanostructure-coated silica microsphere probe. (a) Preparation of silica microsphere on the fibre tip, (b) synthesis and deposition of Ag nanostructures on the microsphere by laser-assisted photochemical method.
Download figure:
Standard image High-resolution imageThe structure of the proposed SERS probe based on a silica microsphere coated by Ag-nanostructures, combined with reflection converging mirror, is shown in figure 2. The exciting laser beam is transmitted through the fibre to the nano-Ag coated microsphere, and one major part of the Raman signal from the nano-Ag layer on the microsphere surface is emitted and focused to the photo-detector through the fibre via a fibre splitter in the Raman equipment. The other part of the Raman signal emitted outside the microsphere is collected by a reflection converging mirror, which has a hemispherical form and suitable diameter for focusing the Raman signal back into the fibre. The distance between the mirror and microsphere will be corrected by an adjustment crew to collectthe maximum Raman intensity and minimum pumping laser beam back to the photo-detector. In order to investigate the performance of the SERS-activity of the microsphere probes, we used low concentrations of R6G solutions. The R6G (Sigma-Aldrich) was diluted into deionized water to make aqueous solutions with different R6G concentrations ranging from 10−4–10−10 M. 2 μl droplets of the R6G-diluted solutions were dropped on the SERS microsphere probes and the testing solutions were naturally dried in atmospheric conditions to adsorb the R6G molecules onto the Ag nanostructures, followed by closing the reflection converging mirror to the SERS probe. The Raman scattering measurements were performed at room temperature with a laser excitation wavelength of 532 nm transmitted through the fibre, and the spectrum acquisition time was 10 s.
Figure 2. Structure of the SERS probe using nano-Ag coated silica microsphere and reflection converging mirror. The dashed-lines denote the excited laser beam, and the solid-lines are the Raman signal.
Download figure:
Standard image High-resolution image3. Results and discussion
Figure 3 presents a schematic of a distribution of an excited laser beam at the end facet of the fibre and at the microsphere, and the SEM images of Ag-nanostructures areas deposited on the end facet of the optical fibre with a core diameter of 50 microns and on the surface of microsphere made from silica fibre with the same diameter.
Figure 3. Schematic distribution of laser intensity and the SEM images of Ag-nanostructure deposition area on the end facet of the optical fibre (a), and on microsphere surface (b), the morphology of Ag-nanodendrite (c), and EDX spectrum recorded on the Ag-deposited on the silica microsphere (d).
Download figure:
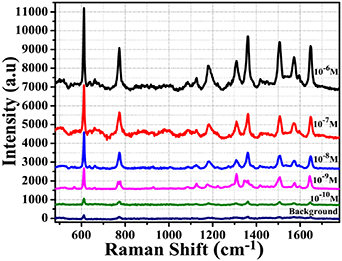
Standard image High-resolution imageIt can be observed that the Ag-nanostructure area demonstrated growth and deposition of Ag-nanostructures occur only in the light-irradiation area. Because of the optical gradient force on the irradiated surface induced by the green laser beam, suspended Ag-nano particles are quickly driven toward the region of the intensive optical field and are finally attached to the silica microsphere surface [19]. In addition, the metallic-nano structures may be attached to the surface of the silica microsphere by electrostatic field [25]. Figure 3(a) shows the Ag-nanostructure-coated area with the broken part in the centre on the end facet of the fibre, which is caused by the high-sharp Gaussian distribution of irradiation laser intensity through the fibre after laser irradiation of 12 min. Figure 3(b) presents the Ag-nano layer that was more homogenously distributed on the silica microsphere surface after laser irradiation of 15 min with the same laser irradiation intensity for the case of the end facet of the fibre; the diameter of the Ag-nanostructure area deposited on the microsphere surface was 80 μm, when the core diameter of the transmittingfibre was 50 microns. The homogeneous distribution of Ag-nanodendrites on the microsphere can be explained by the low-sharp Gaussian distribution of laser intensity in the irradiation area on the microsphere surface in comparison to the fibre end facet. The morphology of Ag-nanodendrite is shown in figure 3(c). The length of Ag-dendrites was 1 μm and their branches were 50–150 nm. Figure 3(d) presents the components of the SERS probe analysed by the EDX spectrum. The peaks of O, Ge and Si correspond to the chemical elements of a typical germanium-doped silica optical fibre, and the peak Ag definitively presents Ag nanostructures growth on the surface of the microsphere. The SERS-activity of Ag-nanostructures, which have grown and been deposited on the surface of the silica microsphere probe, was carried out with the diluted R6G solution using the Raman system (Horiba Scientific LabRam HR Evolution). Figure 4 illustrates the SERS spectra of R6G solution with ultralow concentrations ranging from 10−6 M to 10−10 M dispersed onto Ag-nanostructure-coated microsphere probes produced by the same synthesis process.
Figure 4. Raman spectra of R6G solutions with concentrations ranging from 10–6 M to 10–10 M measured by proposed SERS probe. The background curve was measured by normal Raman technique from 1 M concentration of R6G.
Download figure:
Standard image High-resolution imageIn all measurements, the same 2 μl volume of R6G solution was used. Experiments show that the intensity of the SERS signal increases as the concentration of R6G increases. However, normal Raman signals of R6G are hard to acquire at low concentrations (e.g. 10−6 M) by a silica microsphere without Ag-nanostructures, so an R6G with a high concentration of 1 M was applied to obtain the normal Raman spectrum (see line 'Background line' in figure 4).
The Raman peak bands can be clearly observed in solutions with R6G concentrations down to 10−10 M. Raman spectra of R6G-adsorbed Ag nanostructures were clearly observed, with the feature characteristic peaks at around 611.6 cm−1 associated with C-C-C ring in-plane, 774.1 cm−1 assigned to out-of-plane bending, 1181.9 cm−1 corresponding to the C-H in-plane bending vibrations, and 1308.4 cm−1, 1361.3 cm−1, 1508.5 cm−1, 1573.6 cm−1 and 1645.4 cm−1 corresponding to symmetric modes of C–C in-plane stretching vibrations, which are in good agreement with the literature [26].
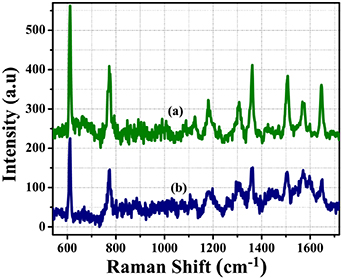
The effect of the reflection converging mirror for collection of Raman intensity was verified by the detection of R6G at a concentration of 10−4 M for both SERS probes within and/or without reflection converging mirror, as shown in figure 5. The intensity of major Raman peaks of R6G with a concentration of 10−4 M measured by SERS probe based on an Ag-nanostructure-coated microsphere and reflection converging mirror is increased up to 32% in comparison with measured results using only an Ag-nanostructure-coated microsphere.
Figure 5. Raman spectra of R6G with concentration of 10–4 M measured by Ag-nanostructure-coated microsphere within (a) and without (b) reflection converging mirror.
Download figure:
Standard image High-resolution imageThe comparison between the Raman spectra without SERS of concentrations of 1 M R6G and with SERS of concentration of 10−10 M is shown in figure 6. The Ag-nanostructure-coated silica exhibits excellent SERS-activity for all major peak positions of R6G at ultralow concentrations of 10−10 M. To quantitatively estimate the Raman activity of the SERS probe based on Ag-nanostructures coated on silica microspheres combined with a reflection converging mirror, we calculate the EF according to the formula [27]:

Figure 6. Raman spectra of R6G solutions with concentration of 10–10 M measured by SERS substrate (a) and with 1 M concentration measured by normal Raman technique (b).
Download figure:
Standard image High-resolution imagewhere ISERS represents the enhanced Raman intensity of R6G adsorbed onto the Ag-nanostructure functionalized microsphere probe and IR represents normal Raman intensity (non-SERS) of R6G on a silica microsphere probe without Ag-nanostructure; CSERS and CR are the concentration of R6G in SERS spectrum and in normal Raman spectrum, respectively. The values of EF for major Raman peaks of R6G of the SERS microsphere probe measured on the R6G concentration of 10−10 M is shown in table 1.
Table 1. Values of EF for major Raman peaks of R6G at a concentration of 10–10 M on the proposed SERS probe.
| Peak position (cm−1) | 611.6 | 774.1 | 1361.3 | 1508.5 | 1645.4 |
|---|---|---|---|---|---|
| Enhancement factor | 1.80 × 1010 | 1.72 × 1010 | 1.67 × 1010 | 1.59 × 1010 | 1.66 × 1010 |
The EF value of the proposed SERS probe is very high in comparison with EF values of nano-Au and/or Ag:Au bimetallic nano-dendrites, as reported in [28, 29]. It is remarkable that the EF value of 107 may be sufficient for single-molecule detection [30], so that our SERS substrate based on a nano-Ag-coated silica microsphere combined with reflection converging mirror can be used for detection of biochemical compounds with ultralow concentrations in the range of part-per-billion (ppb) in liquid environments.
4. Conclusions
In summary, we have proposed a novel SERS probe based on Ag nanostructures coated on the surface of a silica microsphere combined with a reflection converging mirror, which has a SERS-activity probe with a very high enhancement factor of 1.8 × 1010 for the detection of R6G with ultralow concentrations down to 10−10 M. The SERS probe has been shown to have a significant increase of collected Raman signal up to 32% by using a reflection converging mirror in comparison with a non-reflection mirror. The SERS probe can be used as a good quality sensor for the detection of biochemical compounds with ultralow concentrations in liquid environments.
Acknowledgments
This work is financially supported by the NAFOSTED project No.103.03-2018.306 and a part of the VAST project under Grant No.UDPTCN02/19-21.