Abstract
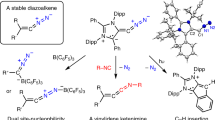
In contrast to naturally occurring F2, O2 and N2, diatomic C2 is an intriguing species that has only been observed indirectly in the gas phase, and because of its high reactivity has eluded isolation in the condensed phase. It has previously been stabilized in L→C2←L compounds but the bonding situation of the central C2 in this motif differs remarkably from that of free C2. Here we have prepared and structurally characterized diatomic C2 as a monoligated complex L→C2 using a bulky phosphine ligand bearing two imidazolidin-2-iminato groups (L is (NHCR=N)2(CH3)P, where NHCR is an N-heterocyclic carbene). The compound is stable in solution at ambient temperature and has also been isolated in the solid state. Reactivity studies, in combination with quantum chemical analysis, suggest that the two carbon atoms of the L→C2 complex both have carbene character. The complex underwent intermolecular C–H bond activation upon thermolysis and exhibited hydroalkoxylation-like reactivity with methanol.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Crystallographic data for the structures in this paper have been deposited at the Cambridge Crystallographic Data Centre under reference numbers 1993538 (2), 1993539 (6), 1993540 (4), 1993541 (3) and 1993542 (5). Copies of the data can be obtained free of charge from www.ccdc.cam.ac.uk/structures/. All other data supporting the findings of this study are available within the article and its Supplementary Information, or from the corresponding authors upon reasonable request.
References
Skell, P. S. & Harris, R. F. Some chemistry of the C2 molecule. J. Am. Chem. Soc. 88, 5933–5934 (1966).
Skell, P. S. & Plonka, J. H. Chemistry of the singlet and triplet C2 molecules. Mechanism of acetylene formation from reaction with acetone and acetaldehyde. J. Am. Chem. Soc. 92, 5620–5624 (1970).
Swan, W. On the prismatic spectra of the flames of compounds of carbon and hydrogen. Trans. R. Soc. Edinb. 21, 411–430 (1857).
Kopfermann, H. & Schweitzer, H. A band system of diatomic carbon vapour. Z. Phys. 61, 87–94 (1930).
Danovich, D., Hiberty, P. C., Wu, W., Rzepa, H. S. & Shaik, S. The nature of the fourth bond in the ground state of C2: the quadruple bond conundrum. Chem. Eur. J. 20, 6220–6232 (2014).
Hermann, M. & Frenking, G. The chemical bond in C2. Chem. Eur. J. 22, 4100–4108 (2016).
Shaik, S., Danovich, D., Braida, B. & Hiberty, P. C. The quadruple bonding in C2 reproduces the properties of the molecule. Chem. Eur. J. 22, 4116–4128 (2016).
Frenking, G. & Hermann, M. Comment on ‘the quadruple bonding in C2 reproduces the properties of the molecule’. Chem. Eur. J. 22, 18975–18976 (2016).
Shaik, S., Danovich, D., Braida, B. & Hiberty, P. C. A response to a comment by G. Frenking and M. Hermann on: ‘the quadruple bonding in C2 reproduces the properties of the molecule’. Chem. Eur. J. 22, 18977–18980 (2016).
Li, Y. et al. C4 cumulene and the corresponding air-stable radical cation and dication. Angew. Chem. Int. Ed. 53, 4168–4172 (2014).
Jin, L., Melaimi, M., Liu, L. & Bertrand, G. Singlet carbenes as mimics for transition metals: synthesis of an air stable organic mixed valence compound [M2(C2)+·; M = cyclic(alkyl)(amino)carbene]. Org. Chem. Front. 1, 351–354 (2014).
Wu, D., Li, Y., Gangulyb, R. & Kinjo, R. Synthesis and structural characterization of a C4 cumulene including 4-pyridylidene units, and its reactivity towards ammonia-borane. Chem. Commun. 50, 12378–12381 (2014).
Georgiou, D. C. et al. The fate of NHC-stabilized dicarbon. Chem. Eur. J. 21, 3377–3386 (2015).
Wang, Y. et al. A stable silicon(0) compound with a Si=Si double bond. Science 321, 1069–1071 (2008).
Sidiropoulos, A., Jones, C., Stasch, A., Klein, S. & Frenking, G. N-Heterocyclic carbene stabilized digermanium(0). Angew. Chem. Int. Ed. 48, 9701–9704 (2009).
Jones, C., Sidiropoulos, A., Holzmann, N., Frenking, G. & Stasch, A. An N-heterocyclic carbene adduct of diatomic tin, :Sn=Sn. Chem. Commun. 48, 9855–9857 (2012).
Bestmann, H.-J. et al. Triphenylphosphonioacetylide: a species isoelectronic with isocyanides. Angew. Chem. Int. Ed. 37, 338–342 (1998).
Goldberg, S. Z., Duesler, E. N & Raymond, K. N. Crystal and molecular structure of [Mn(CO)4(C2PPh3)Br]—a co-ordination compound of the unsual carbonyl-ylide product, Ph3P+–C≡C:–. Chem. Commun. 826–827 (1971).
Asay, M., Donnadieu, B., Schoeller, W. W. & Bertrand, G. Synthesis of allenylidene lithium and silver complexes, and subsequent transmetalation reactions. Angew. Chem. Int. Ed. 48, 4796–4799 (2009).
Wu, X. & Tamm, M. Transition metal complexes supported by highly basic imidazolin-2-iminato and imidazolin-2-imine N-donor ligands. Coord. Chem. Rev. 260, 116–138 (2014).
Ochiai, T., Franz, D. & Inoue, S. Applications of N-heterocyclic imines in main group chemistry. Chem. Soc. Rev. 45, 6327–6344 (2016).
Dielmann, F. et al. A crystalline singlet phosphinonitrene: a nitrogen atom-transfer agent. Science 337, 1526–1528 (2012).
Jacobsen, N. E. NMR Spectroscopy Explained: Simplified Theory, Applications and Examples for Organic Chemistry and Structural Biology (John Wiley, 2007).
Chivers, T., Doxsee, D. D., Fait, J. F. & Parvez, M. Preparation and solid-state isomerization of Se,Se′-dialkyltetraphenyldiphosphadiselenatetrazocines: X-ray structures of 1,5-Ph4P2N4Se2Me2 and [Ph2P(NH2)2]2Se. Inorg. Chem. 32, 2243–2248 (1993).
Huber, K.P. & Herzberg, G. Molecular Spectra and Molecular Structure IV. Constants of Diatomic Molecules (Van Nostrand-Reinhold, 1990).
Weast, R. C., Astle, M. J. & Beyer, W. H. CRC Handbook of Chemistry and Physics 65th edn (CRC Press, 1984).
Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts 3rd edn (John Wiley, 2004).
Haaland, A. Covalent versus dative bonds to main group metals, a useful distinction. Angew. Chem., Int. Ed. 28, 992–1007 (1989).
Zhao, L., Hermann, M., Holzmann, N. & Frenking, G. Dative bonding in main group compounds. Coord. Chem. Rev. 344, 163–204 (2017).
Michalak, A., Mitoraj, M. & Ziegler, T. J. Bond orbitals from chemical valence theory. Phys. Chem. A. 112, 1933–1939 (2008).
Zhao, L., Hermann, M., Schwarz, W. H. E. & Frenking, G. The Lewis electron-pair bonding model: modern energy decomposition analysis. Nat. Chem. Rev. 3, 48–63 (2019).
Weinstein, C. M. et al. Highly ambiphilic room temperature stable six-membered cyclic (alkyl)(amino)carbenes. J. Am. Chem. Soc. 140, 9255–9260 (2018).
Chan, Y.-C. et al. A dimeric NHC–silicon monotelluride: synthesis, isomerization, and reactivity. Angew. Chem. Int. Ed. 38, 11723–11727 (2017).
Wünsche, M. A. et al. Imidazol-2-ylidenaminophosphines as highly electron-rich ligands for transition-metal catalysts. Angew. Chem. Int. Ed. 54, 11857–11860 (2015).
Acknowledgements
This research was supported in part by the Ministry of Science & Technology of Taiwan (108-2113-M-001-026-MY3), an Academia Sinica Investigator Award Grant (AS-IA-108-M04), the National Natural Science Foundation of China (grant numbers 21703099, 21973044 and 21828101), the Natural Science Foundation of Jiangsu Province for Youth (grant number BK20170964) and Nanjing Tech University (grant numbers 39837123 and 39837132). We are grateful to the High-Performance Computing Center of Nanjing Tech University for supporting the computational resources. T.Y. acknowledges financial support from the Alexander von Humboldt foundation. Further support came from the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Contributions
T.-F.L. conceived the study. T.-F.L. and M.-C.W. performed and studied experiments. W.-M.C. assisted with the infrared measurements. D.J., D.X. and T.Y. carried out computational calculations. G.P.A.Y. did the crystallographic work. T.-F.L., D.J. and M.-C.W. contributed equally to the work and all authors contributed to data analysis. T.-G.O, L.Z. and G.F. supervised research and acquired funding. T.-F.L. and T.-G.O. wrote the original draft, which was reviewed and edited with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Data 1
CIF for compound 2.
Supplementary Data 2
CIF for compound 3.
Supplementary Data 3
CIF for compound 4.
Supplementary Data 4
CIF for compound 5.
Supplementary Data 5
CIF for compound 6.
Rights and permissions
About this article
Cite this article
Leung, TF., Jiang, D., Wu, MC. et al. Isolable dicarbon stabilized by a single phosphine ligand. Nat. Chem. 13, 89–93 (2021). https://doi.org/10.1038/s41557-020-00579-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-00579-w
This article is cited by
-
A silylene-stabilized ditin(0) complex and its conversion to methylditin cation and distannavinylidene
Nature Communications (2023)