Abstract
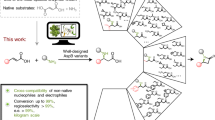
Expanding the toolbox of enzymatic reactions accessible to organic chemists is one of the major goals in biocatalysis. Here we describe the development of an acyltransferase variant from Mycobacterium smegmatis in which a strategic Ser/Cys exchange in the catalytic triad dramatically expanded its synthetic capability to yield a biocatalyst able to efficiently catalyse the formation of thioesters and tertiary amides in water. Preparative scale (250 mM) biotransformations were performed starting from different thiols and secondary amines with excellent yields and reactions times, using vinyl esters as acylating agents. The high substrate-to-catalyst ratio and the cofactor independence make this process a sustainable and cost-effective procedure that was successfully applied to the synthesis of acetyl coenzyme A as well as structurally simpler analogues. Computational studies provided insights into the enzymatic selectivity and substrate recognition.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data are available from the corresponding authors upon request. The crystal structure used as the starting point for the in silico analysis is available in the Protein Data Bank (https://www.rcsb.org/) under accession no. 2Q0S. The dataset for conservation analysis can be found in https://pfam.xfam.org/family/PF13472.
References
Sheldon, R. A. & Pereira, P. C. Biocatalysis engineering: the big picture. Chem. Soc. Rev. 46, 2678–2691 (2017).
Devine, P. N. et al. Extending the application of biocatalysis to meet the challenges of drug development. Nat. Rev. Chem. 2, 409–421 (2018).
Jordan, A. & Sneddon, H. F. Development of a solvent–reagent selection guide for the formation of thioesters. Green Chem. 21, 1900–1905 (2019).
Petchey, M. R. & Grogan, G. Enzyme-catalysed synthesis of secondary and tertiary amides. Adv. Synth. Catal. 361, 3895–3914 (2019).
YI, C. L., Huang, Y. T. & Lee, C. F. Synthesis of thioesters through copper catalyzed coupling of aldehydes with thiols in water. Green Chem. 15, 2476–2484 (2013).
Hirschbeck, V., Gehrtz, P. H. & Fleischer, I. Metal-catalyzed synthesis and use of thioesters: recent developments. Chem. Eur. J. 24, 7092–7107 (2018).
Pattabiraman, R. V. & Bode, J. W. Rethinking amide bond synthesis. Nature 480, 471–479 (2011).
Contente, M. L., Pinto, A., Molinari, F. & Paradisi, F. Biocatalytic N-acylation of amines in water using an acyltransferase from Mycobacterium smegmatis. Adv. Synth. Catal. 360, 4814–4819 (2018).
Lustosa de Melo Carvalho, A. C. et al. Recent advances in lipase-mediated preparation of pharmaceuticals and their intermediates. Int. J. Mol. Sci. 16, 29682–29716 (2015).
Lou, F. W., Liu, B. K., Wang, J. L., Pan, Q. & Lin, X. F. Controllable enzymatic Markovnikov addition acylation of thiols to vinyl esters. J. Mol. Catal. B 60, 64–68 (2009).
Zhou, N. et al. Enzymatic synthesis of thioesters from thiols and vinyl esters in a continuous-flow microreactor. Catalysts 8, 249–261 (2018).
Balessari, A. & Mangone, C. P. One-pot biocatalyzed preparation of substituted amides as intermediates of pharmaceuticals. J. Mol. Catal. B 11, 335–341 (2001).
Wood, A. J. L. et al. Adenylation activity of carboxylic acid reductases enables the synthesis of amides. Angew. Chem. 129, 14690–14693 (2017).
Philpott, H. K., Thomas, P. J., Tew, D., Fuerst, D. E. & Lovelock, S. L. A versatile approach to amide bond formation. Green Chem. 20, 3426–3431 (2018).
Mathews, I. et al. Structure of a novel enzyme that catalyzes acyl transfer to alcohols in aqueous conditions. Biochemistry 46, 8969–8979 (2007).
Wiermans, L. et al. Transesterifications and peracid-assisted oxidations in aqueous media catalyzed by Mycobacterium smegmatis acyl transferase. ChemCatChem 5, 3719–3724 (2013).
Perdomo, I. et al. Efficient enzymatic preparation of flavor esters in water. J. Agr. Food Chem. 67, 6517–6522 (2019).
Contente, M. L., Farris, S., Tamborini, L., Molinari, F. & Paradisi, F. Flow-based enzymatic synthesis of melatonin and other high value tryptamine derivatives: a five-minute intensified process. Green Chem. 21, 3263–3266 (2019).
Contente, M. L., Tamborini, L., Molinari, F. & Paradisi, F. Aromas flow: eco-friendly, continuous, and scalable preparation of flavour esters. J. Flow. Chem. 10, 235–240 (2020).
de Leeuw, N. et al. Ester synthesis in water: Mycobacterium smegmatis acyl transferase for kinetic resolution. Adv. Synth. Catal. 360, 242–249 (2018).
Finnveden, M., Semlitsch, S., He, O. & Martinelle, M. Mono-substitution of symmetric diesters: selectivity of Mycobacterium smegmatis acyltransferase variants. Catal. Sci. Technol. 9, 4920–4927 (2019).
Kazemi, M., Sheng, X., Kroutil, W. & Himo, F. Computational study of Mycobacterium smegmatis acyl transferase reaction mechanism and specificity. ACS Catal. 8, 10698–10706 (2018).
Neet, K. E. & Koshland, D. E. Jr. The conversion of serine at the active site of subtilisin to cysteine: a ‘chemical mutation’. Proc. Natl Acad. Sci. USA 56, 1606–1611 (1966).
Polgar, L. & Bender, M. L. A new enzyme containing a synthetically formed active site thiol-subtilisin. J. Am. Chem. Soc. 88, 3153–3154 (1966).
Higaki, J. N., Evnin, L. B. & Craik, C. S. Introduction of a cysteine protease active site into trypsin. Biochemistry 28, 9256–9263 (1989).
Wilke, M., Higaki, J. N., Craik, C. S. & Fletterick, R. J. Crystal structure of rat tripsin-S195C at –150 °C: analysis of low activity of recombinant and semisynthetic thiol protease. J. Mol. Biol. 219, 511–523 (1991).
Cen, Y. et al. Artificial cysteine-lipases with high activity and altered catalystic mechanism created by laboratory evolution. Nat. Commun. 10, 1–10 (2019).
Staunton, J. & Weissman, K. Polyketide biosynthesis: a millennium review. J. Nat. Prod. Rep. 18, 380–416 (2001).
Mercer, A. C. & Burkart, M. D. The ubiquitous carrier protein—a window to metabolite biosynthesis. Nat. Prod. Rep. 24, 750–773 (2007).
Franke, J. & Hertweck, C. Biomimetic thioesters as probes for enzymatic assembly lines: synthesis, applications and challenges. Cell Chem. Biol. 23, 1179–1192 (2016).
Agarwal, V. et al. Chemoenzymatic synthesis of acyl coenzyme A substrates enables in situ labelling of small molecules and proteins. Org. Lett. 17, 4452–4455 (2015).
Torres, P. H. M., Sodero, A. C. R., Jofily, P. & Silva-Jr, F. P. Key topics in molecular docking for drug design. Int. J. Mol. Sci. 20, 4574 (2019).
Miller, B. R. et al. MMPBSA.py: an efficient program for end-state free energy calculations. J. Chem. Theory Comput. 8, 3314–3321 (2012).
Wang, E. et al. End-point binding free energy calculation with MM/PBSA and MM/GBSA: strategies and applications in drug design. Chem. Rev. 119, 9478–9508 (2019).
Vávra, O. et al. CaverDock: a molecular docking-based tool to analyse ligand transport through protein tunnels and channels. Bioinformatics 35, 1–8 (2019).
Filipovic, J. et al. CaverDock: a novel method for the fast analysis of ligand transport. IEEE/ACM Trans. Comput. Biol. Bioinf. https://doi.org/10.1109/TCBB.2019.2907492 (2019).
Godehard, S. P., Badenhorst, C. P. S., Müller, H. & Bornscheuer, U. T. Protein engineering for enhanced acyltransferase activity, substrate scope, and selectivity of the Mycobacterium smegmatis acyltransferase MsAcT. ACS Catal. 10, 7552–7562 (2020).
Land, H., Hendil-Forssell, P., Martinelle, M. & Berglund, P. One-pot biocatalytic amine transaminase/acyl transferase cascade for aqueous formation of amides from aldehydes or ketones. Catal. Sci. Technol. 6, 2897–2900 (2016).
Acknowledgements
This project was supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 792804 AROMAs-FLOW (M.L.C.), and SNSF (200021_192274, F.P.). The authors thank P. Renaud (DCB, University of Bern) for insightful scientific discussions.
Author information
Authors and Affiliations
Contributions
F.P. developed the original concept. M.L.C. performed the experimental work and analysed the results. D.R.-P. conceived and performed the computational experiments. M.L.C., F.M. and F.P. conceived and designed the experiments. All the authors co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks Luis Morales-Quintana, Nicholas Turner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–9 and references.
Supplementary Video 1
Depiction of the transport of CoA from the bulk to the active site and acetyl CoA back outside in the tunnel identified.
Supplementary Data 1
Initial and final coordinates of the systems in the molecular dynamics simulations as pdb formatted files.
Rights and permissions
About this article
Cite this article
Contente, M.L., Roura Padrosa, D., Molinari, F. et al. A strategic Ser/Cys exchange in the catalytic triad unlocks an acyltransferase-mediated synthesis of thioesters and tertiary amides. Nat Catal 3, 1020–1026 (2020). https://doi.org/10.1038/s41929-020-00539-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-00539-0
This article is cited by
-
Thioester-mediated biocatalytic amide bond synthesis with in situ thiol recycling
Nature Catalysis (2022)