Abstract
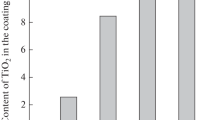
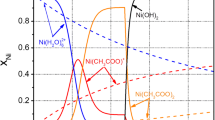
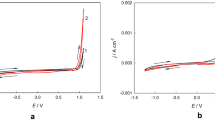
The process of electrodeposition of nickel–titanium dioxide composite coatings from an electrolyte based on a choline-containing ionic liquid was studied. To increase the content of the dispersed phase in the composite, it is appropriate to add water to the electrolyte. With inclusion of the titanium dioxide particles into a nickel matrix, the microhardness of the coating increases. The obtained coatings show electrocatalytic activity in the reactions of hydrogen and oxygen evolution in an alkaline aqueous solution, which can be used in electrolytic decomposition of water.




Similar content being viewed by others
REFERENCES
Plechkova, N.V. and Seddon, K.R., Chem. Soc. Rev., 2008, vol. 37, pp. 123–150. https://doi.org/10.1039/b006677j
Abbott, A.P. and McKenzie, K.J., Phys. Chem. Chem. Phys., 2006, vol. 8, no. 37, pp. 4265–4279. https://doi.org/10.1039/B607329H
Abbott, A.P., Ryder, K.S., and König, U., Trans. Inst. Met. Finish., 2008, vol. 86, no. 4, pp. 196–204. https://doi.org/10.1179/174591908X327590
Tomé, L.I.N., Baião, V., da Silva, W., and Brett, C.M.A., Appl. Mater. Today, 2018, vol. 10, pp. 30–50. https://doi.org/10.1016/j.apmt.2017.11.005
Smith, E.L., Abbott, A.P., and Ryder, K.S., Chem. Rev., 2014, vol. 114, no. 21, pp. 11060–11082. https://doi.org/10.1021/cr300162p
Abbott, A.P., Capper, G., Davies, D.L., Rasheed, R.K., and Tambyrajah, V., Chem. Commun., 2003, no. 1, pp. 70–71. https://doi.org/10.1039/B210714G
Danilov, F.I., Protsenko, V.S., Kityk, A.A., Shaiderov, D.A., Vasil’eva, E.A., Pramod Kumar, U., and Joseph Kennady, C., Prot. Met. Phys. Chem. Surf., 2017, vol. 53, no. 6, pp. 1131–1138. https://doi.org/10.1134/S2070205118010203
Thiemig, D., Bund, A., Surf. Coat. Technol., 2008, vol. 202, no. 13, pp. 2976–2984. https://doi.org/10.1016/j.surfcoat.2007.10.035
Chen, W., He, Y., and Gao, W., Surf. Coat. Technol., 2010, vol. 204, no. 15, pp. 2487–2492. https://doi.org/10.1016/j.surfcoat.2010.01.036
Danilov, F.I., Kityk, A.A., Shaiderov, D.A., Bogdanov, D.A., Korniy, S.A., and Protsenko, V.S., Surf. Eng. Appl. Electrochem., 2019, vol. 55, no. 2, pp. 138–149. https://doi.org/10.3103/S106837551902008X
Du, C., Zhao, B., Chen, X.-B., Birbilis, N., and Yang, H., Sci. Rep., , vol. 6, ID 29225. https://doi.org/10.1038/srep2922
Li, R., Dong, Q., Xia, J., Luo, C., Sheng, L., Cheng, F., and Liang, J., Surf. Coat. Technol., 2019, vol. 366, pp. 138–145. https://doi.org/10.1016/j.surfcoat.2019.03.030
Kityk, A.A., Shaiderov, D.A., Vasil′eva, E.A., Protsenko, V.S., and Danilov, F.I., Electrochim. Acta, 2017, vol. 245, pp. 133–145. https://doi.org/10.1016/j.electacta.2017.05.144
Low, C.T.J., Wills, R.G.A., and Walsh, F.C., Surf. Coat. Technol., 2006, vol. 201, nos. 1–2, pp. 371–383. https://doi.org/10.1016/j.surfcoat.2005.11.123
Walsh, F.C. and Ponce de Leon, C., Trans. Inst. Met. Finish., 2014, vol. 92, no. 2, pp. 83–98. https://doi.org/10.1179/0020296713Z.000000000161
Eroglu, D. and West, A.C., J. Electrochem. Soc., 2013, vol. 160, no. 9, pp. D354–D360. https://doi.org/10.1149/2.052309jes
Guglielmi, N., J. Electrochem. Soc., 1972, vol. 119, no. 8, pp. 1009–1012. https://doi.org/10.1149/1.2404383
Vasil’eva, E.A., Smenova, I.V., Protsenko, V.S., Konstantinova, T.E., and Danilov, F.I., Russ. J. Appl. Chem., 2013, vol. 86, no. 11, pp. 1735–1740. https://doi.org/10.1134/S1070427213110177
Maurin, G. and Lavanant, A., J. Appl. Electrochem., 1995, vol. 25, no. 12, pp. 1113–1121. https://doi.org/10.1007/BF00242538
Ahmad, Y.H. and Mohamed, A.M.A., Int. J. Electrochem. Sci., 2014, vol. 9, no. 1, pp. 1942–1963.
Tseluikin, V.N., Prot. Met. Phys. Chem. Surf., 2016, vol. 52, no. 2, pp. 254–266. https://doi.org/10.1134/S2070205116010251
Baghery, P., Farzam, M., Mousavi, A.B., and Hosseini, M., Surf. Coat. Technol., 2010, vol. 204, no. 23, pp. 3804–3810. https://doi.org/10.1016/j.surfcoat.2010.04.061
Wang, W., Hou, F.-Y., Wang, H., and Guo, H.-T., Scr. Mater., 2005, vol. 53, no. 5, pp. 613–618. https://doi.org/10.1016/j.scriptamat.2005.04.002
Hou, F., Wang, W., and Guo, H., Appl. Surf. Sci., 2006, vol. 252, no. 10, pp. 3812–3817. https://doi.org/10.1016/j.apsusc.2005.05.076
Mokabber, T., Rastegari, S., and Razavizadeh, H., Surf. Eng., 2013, vol. 29, no. 1, pp. 41–45. https://doi.org/10.1179/1743294412Y.0000000077
Safizadeh, F., Ghali, E., and Houlachi, G., Int. J. Hydrogen Energy, 2015, vol. 40, no. 1, pp. 256–274. https://doi.org/10.1016/j.ijhydene.2014.10.109
Jamesh, M.I.J., Power Sources, 2016, vol. 333, pp. 213–236. https://doi.org/10.1016/j.jpowsour.2016.09.161
Wang, S., Zou, X., Lu, Y., Rao, S., Xie, X., Pang, Z., Lu, X., Xu, Q., and Zhou, Z., Int. J. Hydrogen Energy, 2018, vol. 43, no. 33, pp. 15673–15686. https://doi.org/10.1016/j.ijhydene.2018.06.188
Kullaiah, R., Elias, L., and Hegde, A.C., Int. J. Miner. Metall. Mater., 2018, vol. 25, no. 4, pp. 472–479. https://doi.org/10.1007/s12613-018-1593-8
Protsenko, V.S., Bogdanov, D.A., Korniy, S.A., Kityk, A.A., Baskevich, A.S., and Danilov, F.I., Int. J. Hydrogen Energy, 2019, vol. 44, no. 45, pp. 24604–24616. https://doi.org/10.1016/j.ijhydene.2019.07.188
Gierlotka, D., Rówiński, E., Budniok, A., and Łagiewka, E., J. Appl. Electrochem., 1997, vol. 27, no. 12, pp. 1349–1354. https://doi.org/10.1023/A:1018416927715
Funding
The study was financially supported by the Ministry of Science and Education of Ukraine (project 0118U003398).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare than they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Protsenko, V.S., Bogdanov, D.A., Kityk, A.A. et al. Ni–TiO2 Functional Composite Coatings Deposited from an Electrolyte Based on a Choline-Containing Ionic Liquid. Russ J Appl Chem 93, 1525–1532 (2020). https://doi.org/10.1134/S1070427220100067
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427220100067