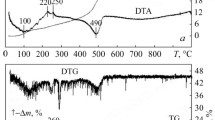
Variations in the phase composition, specific surface area, and morphology of structural components in the ultrafine powder of composition (wt.%) 70 (90 ZrO2 (3 Y2O3, 2 CeO2)–10 Al2O3)–30 CoAl2O4 (70ZA30CoA), produced by hydrothermal synthesis combined with mechanical mixing, were studied in the heat treatment process up to 1300°C. The study employed Xray diffraction, scanning and transmission electron microscopy, petrography, and BET. The formation of CoAl2O4 in the 70ZA30CoA powder in the heat treatment process was accompanied by reversible phase transformations: T-ZrO2 → M-ZrO2 → T-ZrO2. The M-ZrO2 content increased from 15% to 46% in the temperature range 850–1000°C and decreased to 13% after heat treatment to 1150°C. The process involved slight coarsening of the primary T-ZrO2 particles, while the size of the primary M-ZrO2 particles remained practically unchanged. The phase transformation was due to a decrease in the free energy of the ultrafine 70ZA30CoA powder, representing a thermodynamically nonequilibrium system. The phase composition changed color of the 70ZA30CoA powder in the following sequence: gray → gray blue → dark cyan → bright blue. Morphological analysis of the structural components showed that the CoAl2O4 formation and reversible T-ZrO2 → M-ZrO2 phase transformation were accompanied by shape change, loosening, and subsequent sintering of the agglomerates. The chain-like agglomerates of various shapes and sizes indicate that the 70ZA30CoA powder sinters actively at 1300°C. The decrease in the specific surface area from 46 to 1 m2/g depending on the heat treatment temperature was determined by the development of three structural transformation processes: formation of CoAl2O4, phase transition of the ZrO2 solid solution, and sintering of the 70ZA30CoA powder. The established regularities are of fundamental importance for the microstructural design of ZrO2 composites such as ZrO2–Y2O3–CeO2–Al2O3–CoO materials of blue and other colors for various applied purposes.




Similar content being viewed by others
References
R.H.J. Hannink, P.M. Kelly, and B.C. Muddle, “Transformation toughening in zirconia-containing ceramics,” J. Am. Ceram. Soc., 83, No. 3, 461–487 (2000).
G.W. Liu, Z.P. Xie, W. Wang, Y. Wu, and X.F. Yang, “Fabrication of colored zirconia ceramics by infiltrating water debound injection moulded green body,” Adv. Appl. Ceram., 110, No. 1, 58–62 (2011).
E.V. Dudnik, S.N. Lakiza, V.V. Tsukrenko, Ya.S. Tishchenko, A.K. Ruban, and V.P. Redko, “Nanocrystalline oxide powders for microstructural design of materials,” Nanosyst. Nanomater. Nanotechnol., 14, No. 4, 561–575 (2016).
W. Wang, W. Liu, X. Yang, and Zh. Xie, “Fabrication of black-colored CuO–Al2O3–ZrO2 ceramics via heterogeneous nucleation method,” Ceram. Int., 38, 2851–2856 (2012).
M. Matsuda, Y. Himeno, K. Shida, and M. Matsuda, “Black-ZrO2 thin film produced by oxidation of Zr metal plate in air,” Mater. Lett., 230, 117–119 (2018).
H. Lv, J. Bao, F. Ruan, F. Zhou, Q. Wang, W. Zhang, W. Guo, Y. Zhang, X. Song, and Sh. An, “Preparation and properties of black Ti-doped zirconia ceramics. Preparation and properties of black Ti-doped zirconia ceramics. J. Mater. Res. Technol. (2020), URL: https://doi.org/10.1016/j.jmrt.2020.01.021.
Y. Li, M. Wang, H. Wu, F. He, Y. Chenc, and Sh. Wu, “Cure behavior of colorful ZrO2 suspensions during digital light processing (DLP) based stereolithography process,” J. Eur. Ceram. Soc., 39, 4921–4927 (2019).
J.M. Calatayud and J. Alarcón, “V-containing ZrO2 inorganic yellow nano-pigments prepared by hydrothermal approach,” Dyes Pigm., 146, 178–188 (2017).
A.M. Neris, L. Chantelle, J.J. Souza, J.M. Ferreira, M.G. Fonseca, and I.M.G. Santos, “Environmental remediation and synthesis of a new pigment by irradiation-induced adsorption of methylene blue onto undoped tetragonal zirconia,” Mater. Lett., 255, 1–4 (2019).
R. Harada, S. Takemoto, M. Hattori, M. Yoshinari, Y. Oda, and E. Kawada, “The influence of colored zirconia on the optical properties of all-ceramic restorations,” Dent. Mater. J., 34, No. 6, 918–924 (2015).
Ch.-T. Kao, W.-H. Tuan, Ch.-Y. Liu, and Sh.-Ch. Chen, “Effect of iron oxide coloring agent on the sintering behavior of dental yttria-stabilized zirconia,” Ceram. Int., 44, No. 5, 4689–4693 (2018), URL: https://doi.org/10.1016/j.ceramint.2017.12.049.
S.B. Haralur, N.R.S. Alqahtani, and Al.F. Mujayri, “Effect of hydrothermal aging and beverages on color stability of lithium disilicate and zirconia-based ceramics,” Medicina, 55, 749 (2019), URL: doi:https://doi.org/10.3390/medicina 55110749.
E. Kontonasaki, P. Giasimakopoulos, and A.E. Rigos, “Strength and aging resistance of monolithic zirconia: an update to current knowledge,” Jpn. Dent. Sci. Rev., 56, 1–23 (2020).
P.G. Read, “Synthetic gemstones and gemstone simulants,” Gemmology, 193–216 (1991), URL: https://doi.org/10.1016/C2013-0-04574-4. Copyright 1991. Elsevier Ltd. All rights reserved. ISBN 978-0-7506-1066-7.
W. Wang, Zh. Xie, G. Liu, and W. Yang, “Fabrication of blue-colored zirconia ceramics via heterogeneous nucleation method,” Cryst Growth Des., 9, No. 10, 4373–4377 (2009).
I. Yamashita, M. Kudo, and K. Tsukuma, “Development of highly transparent zirconia ceramics,” Tosoh Res. Technol. Rev., 56, 11–16 (2012).
Y.H. Chiou and S.T. Lin, “Influence of CoO and Al2O3 on the phase partitioning of ZrO2–3 mol.% Y2O3,” Ceram. Int., 22, 249–256 (1996).
G.W. Liu, Z.P. Xie, W. Wang, Y. Wu, and X.F. Yang, “Fabrication of colored zirconia ceramics by infiltrating water debound injection moulded green body,” Adv. Appl. Ceram., 110, 58–62 (2011).
A. Yurdakul and H. Gocmez, “One-step hydrothermal synthesis of yttria-stabilized tetragonal zirconia polycrystalline nanopowders for blue-colored zirconia-cobalt aluminate spinel composite ceramics,” Ceram. Int., 45, 5398–5406 (2019).
E.F. Belenkii, Chemistry and Technology of Pigments [in Russian], Gos. Nauch. Tekh. Izd. Khim. Lit., Leningrad (1960), p. 756.
A. Aguilar-Elguezabal, M. Román-Aguirre, L. De la Torre-Sáenz, P. Pizá-Ruiz, and M. Bocanegra-Bernal, “Synthesis of CoAl2O4/Al2O3 nanoparticles for ceramic blue pigments,” Ceram. Int., 43, Issue 17, 15254–15257 (2017).
A.V. Shevchenko, E.V. Dudnik, V.V. Tsukrenko, A.K. Ruban, V.P. Red’ko, and L.M. Lopato, “Microstructural design of bioinert composites in the ZrO2–Y2O3–CeO2–Al2O3–CoO system,” Powder Metall. Met. Ceram., 51, No. 11–12, 724–733 (2013).
E.V. Dudnik, V.V. Tsukrenko, M.S. Glabai, A.K. Ruban, V.P. Red’ko, and A.I. Khomenko, “Nanocrystalline powders in ZrO2–Y2O3–CeO2–Al2O3–CoO system for microstructural design of ZrO2-based color composites,” Powder Metall. Met. Ceram., 56, No. 7–8, 407–415 (2017).
V.V. Skorokhod, “Hierarchy of structural levels and structural engineering of inorganic materials,” in: Inorganic Materials Science [in Russian], Naukova Dumka, Kyiv (2008), Vol. 1, pp. 339–357.
E.V. Dudnik, A.V. Shevchenko, A.K. Ruban, V.P. Red’ko, and L.M. Lopato, “Microstructural design of ZrO2–Y2O3–CeO2–Al2O3 materials,” Powder Metall. Met. Ceram., 49, No. 9–10, 528–536 (2011).
A.V. Shevchenko, “Hydrothermal technology in materials science,” in: Inorganic Materials Science [in Russian], Naukova Dumka, Kyiv (2008), Vol. 2, pp. 272–281.
M.S. Glabai, “Determining conditions for synthesizing the CoAl2O4 powder to develop color ZrO2–Y2O3–CeO2–Al2O3–CoO composites,” in: Current Issues of Physical Materials Science [in Russian], Inst. Probl. Materialoved. NAN Ukrainy, Kyiv (2017), Issue 26, pp. 37–42.
M.S. Glabai, V.P. Red’ko, V.V. Tsukrenko, O.K. Ruban, and O.V. Dudnik, “Synthesis and properties of nanocrystalline 90 wt.% ZrO2 (3Y2O3, 2CeO2)–10 wt.% Al2O3 powders doped by CoAl2O4,” Nanosyst. Nanomater. Nanotekhnol., 15, No. 2, 319–328 (2017).
O.V. Dudnik, M.S. Glabai, Ya.S. Tishschenko, V.P. Red’ko, and O.K. Ruban, “Phase transformations following heat treatment of the fine ZrO2–Y2O3–CeO2–Al2O3–CoO powder,” Adhez. Raspl. Paika Mater., Issue 51, 62–70 (2018).
E.V. Dudnik, V.V. Tsukrenko, A.V. Shevchenko, O.K. Ruban, and L.M. Lopato, “Properties of nanocrystalline ZrO2–Y2O3–CeO2–CoO–Al2O3 powders,” Inorg. Mater., 47, No. 10, 1107–1110 (2011).
A.V. Kurdyumov, “Phase transformations in materials,” in: Inorganic Materials Science [in Russian], Naukova Dumka, Kyiv (2008), Vol. 1, pp. 550–567.
T. Sakuma, “Phase transformation and microstructure of partially-stabilized zirconia,” Trans. Jpn. Inst. Met., 29, 879–893 (1988).
P. Li, I-W. Chen, and L.E. Penner-Hahn, “Effect of dopants on zirconia stabilization-an X-ray absorption study. I. Trivalent dopants,” J. Am. Ceram. Soc., 77, No. 1, 118–128 (1994).
P. Li, I-W. Chen, and J.E. Penner-Hahn, “Effect of dopants on zirconia stabilization-an X-ray absorption study. II. Tetravalent dopants,” J. Am. Ceram. Soc., 77, No. 5, 281–288 (1994).
S. Shukla and S. Seal, “Mechanisms of room temperature metastable tetragonal phase stabilization in zirconia,” Int. Mater. Rev., 50, No. 1, 45–64 (2005).
T.K. Atanasyan, I.G. Gorichev, and E.A. Yakusheva, “Surface phenomena at the oxide/electrolyte interface in acid media,” Inorganic Chemistry [in Russian], Prometei, Moscow (2013), Part I, p. 166.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 59, Nos. 7–8 (534), pp. 3–14, 2020.
Rights and permissions
About this article
Cite this article
Dudnik, E., Glabay, M., Kotko, A. et al. Effect of Heat Treatment on the Physicochemical Properties of Ultrafine ZrO2–Y2O3–CeO2–Al2O3–CoO Powders. Powder Metall Met Ceram 59, 359–367 (2020). https://doi.org/10.1007/s11106-020-00169-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-020-00169-y