Abstract
A three-dimensional (3D) flower-like MoS2/Bi2S3 heterojunction is successfully synthesized through a simple one-step hydrothermal route. The special 3D morphology, achieved by assembling 2D MoS2 nanosheets onto 3D Bi2S3 micro-flowers, helps to promote the photogenerated electron–hole separation and the electronic conduction. Thus, the as-prepared MoS2/Bi2S3 heterojunction exhibits a prominent photocatalysis activity and electrochemical performance. Compared with pure Bi2S3 (11.8%) and MoS2 (49.2%), the heterojunction demonstrates the higher percentage of methylene blue degradation (76.2%). The enhanced photocatalytic activity is attributed to the effective separation of the charge carriers between Bi2S3 and MoS2, which is not possible with individual materials. When the MoS2/Bi2S3 heterojunction is tested as a supercapacitor electrode, it shows an optimum capacitance of 100.2 F/g at a current density of 1 A/g, which is about 22-times higher than that of pure Bi2S3 (4.5 F/g). With the key findings, the potential of metal sulfides heterojunction for multifunctional applications is highly expected.
Similar content being viewed by others
1 Introduction
With the development of modern industry and the aggravation of environmental pollution, the fabrication and utilization of high-efficiency and cost-efficient photocatalysts have attracted worldwide attention due to ever-increasing demands for solving renewable energy and environmental issues [1,2,3]. Metal sulfides have wide applications in environmental photocatalysis due to their narrow band gaps, unique structure, and satisfactory catalytic activity [4,5,6,7,8]. Especially, Bi2S3 with a direct narrow band gap of 1.3–1.7 eV has caused widespread concern in the field of photocatalysis based on its unique electric and optical properties [9, 10]. However, the photocatalytic activity of pure Bi2S3 is significantly lower than expected due to its severe charge recombination and structural photocorrosion [11].
Currently, the construction of heterojunctions has been considered as an effective method to realize efficient electron–hole separation and couple respective advantages of each component, which may further enhance the photocatalytic properties of pure Bi2S3 [12, 13]. As a typical layered metal sulfide, MoS2 with two-dimensional (2D) layered structures provides a large number of active sites for interfacial adsorption, convenient electron transfer, and huge surface area to make MoS2 high photocatalytic activity[11]. Therefore, combining MoS2 with Bi2S3 to form the heterojunctions can obtain higher photocatalytic activity than MoS2 or Bi2S3 alone. Until now, there are several reports on MoS2/Bi2S3 heterojunction photocatalyst. Weng et al. [14] used Bi2WO6 discoids as a precursor to prepare 2D MoS2 nanosheets-coated Bi2S3 discoids composites, which exhibits improved photoreduction efficiency of Cr(VI) compared with pure MoS2 and Bi2S3. Luo et al. [15] reported a two-step solvothermal method to synthetic CF/MoS2/Bi2S3 cloth, which exhibits high removal efficiency of dye wastewater. The heterojunction synthesized by the above method has higher photocatalytic performance, but the methods for its preparation are complex and need to be completed in multiple steps, which limits its practical application19. Supercapacitors, as renewable energy storage devices, have become more significant because of their longer operating lifespans, fast charge and discharge, and large power capability [16, 17]. Bi2S3 as an important semiconductor material is considered to be a promising electrode material for supercapacitors ascribed to its unique physical and chemical properties [18]. Besides, MoS2 with a typical layered structure can provide a relatively high theoretical specific capacitance, which is also employed to develop supercapacitor electrodes [19, 20]. However, there are few reports on the MoS2/Bi2S3 heterojunction with dual applications in both photocatalysis and electrode of supercapacitors, simultaneously. The photocatalytic activity and electrochemical performance of MoS2/Bi2S3 heterojunction have not been further explored. Therefore, developing a simple method to synthesize heterojunction and further expanding the versatility of heterojunction are of considerable interest.
In this work, we develop a facile and simple one-step hydrothermal route to synthesize 3D flower-like MoS2/Bi2S3 heterojunction with dual function of both pollutants degradation through photocatalyst and energy storage. The unique heterostructure is beneficial to realize the photogenerated electron–hole separation and improve the electronic conduction; thus, the as-prepared MoS2/Bi2S3 exhibits a prominent photocatalytic activity and electrochemical performance. As a proof of concept, it has been testified that the heterojunction shows higher photocurrent and photocatalytic activity for the degradation of methylene blue under visible light irradiation compared with single pure substance. Moreover, the possible photocatalytic reaction mechanism is also investigated deeply in this work. It is also found that the MoS2/Bi2S3 heterojunction exhibits a relatively high specific capacitance concerning pure Bi2S3. The dual utility for both pollutants degradation through photocatalyst and energy storage through supercapacitors further demonstrates the advantage of the unique 3D flower-like MoS2/Bi2S3 heterojunction.
2 Experimental section
2.1 Synthesis of MoS2/Bi2S3 heterojunction
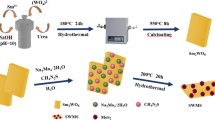
0.2 g bismuth nitrate pentahydrate (Bi(NO3)3·5H2O) was dissolved in 10 mL deionized water, and then 0.17 mL ethylenediamine (C2H8N2) and 0.025 g citric acid monohydrate (C6H8O7·H2O) were added to this solution, then keep stirring for 10 min. Another solution was prepared by dissolving a defined dosage of ammonium molybdate tetrahydrate ((NH4)6Mo7O24·4H2O) and 0.0848 g L-Cysteine (C3H7NO2S) in 5 mL deionized water. Then the as-prepared (NH4)6Mo7O24·4H2O mixed solution was added dropwise to the Bi(NO3)3·5H2O mixed solution, and the final solution was poured to a Teflon-lined stainless steel autoclave and heated to 200 °C for 24 h. After cooled down to room temperature, the synthetic samples were washed with deionized water and ethanol alternately three times and then dried at 60 °C for 12 h. The sample prepared with (NH4)6Mo7O24·4H2O dosages of 0.012 g, 0.025 g, 0.037 g, and 0.062 g were designated as MoS2/Bi2S3-1, MoS2/Bi2S3-2, MoS2/Bi2S3-3, MoS2/Bi2S3-5. Besides, pure MoS2 and Bi2S3 were prepared by a similar procedure and condition without adding Bi(NO3)3·5H2O or (NH4)6Mo7O24·4H2O, respectively.
2.2 Materials characterization
The microstructure and morphology of MoS2/Bi2S3 heterojunction were performed using a field emission scanning electron microscopy (FESEM, Hitachi SU8010) and high-resolution transmission electron microscopy (HRTEM, Tecnai G2 F30). X-ray diffraction (XRD, Bruker D8 Advance) was employed to verify the composition of the sample. X-ray photoelectron spectroscopy (XPS, Escalab 250 Xi) was used to characterize the surface chemistry and bonding regularity of the sample. The UV-Vis diffuse reflectance (DRS, Hitachi U-3900) of the samples was recorded using BaSO4 as reference.
2.3 Photocatalytic evaluation
Methylene blue (MB) was used to evaluate the photocatalytic activity of the MoS2/Bi2S3 heterojunction via visible light degradation. A 300 W xenon lamp equipped with a pass filter (λ > 420 nm) was used as a simulated light source. 10 mg photocatalyst powders were dropped into 50 mL MB solution (10 mg/L). Before irradiation, the above mixture was sonicated for 10 min and then stirred for 60 min in dark to achieve an adsorption-desorption equilibrium between the photocatalyst and MB. During the irradiation reaction, 3 mL supernatant of the mixture was sampled every 1 h. The amounts of MB in the mixture were measured by a UV–Vis spectrophotometer (Hitachi U-3900) with the maximum absorption ban at 664 nm.
2.4 Electrochemical measurements
The electrochemical properties of the samples were measured through an electrochemical workstation (CHI 760E) with a three-electrode system in a 1 M KCl aqueous solution, which consisted of the sample as the working electrode, Ag/AgCl as the reference electrode, and Pt wire as the counter electrode. The working electrodes were prepared as follows: the samples, acetylene black, and polyvinylidene fluoride (PVDF) at a weight ratio of 8:1:1 with N-methyl-2-pyrrolidone (NMP) to form a slurry. The slurry to Ni form is applied to form the working electrodes, and then the prepared electrodes in an oven at 60°C are dried overnight to remove the solvent. The cyclic voltammetry (CV) curves were measured under different scan rates from 10 to 40 mV/s between − 0.9 to − 0.3 V. The galvanostatic charging-discharging (GCD) tests were carried out at a density of 1 to 5 A/g.
3 Results and discussion
3.1 Morphology and structure of MoS2/Bi2S3 heterojunction
The probable morphology evolution process of the flower-like MoS2/Bi2S3 heterojunction is shown in Fig. 1. According to the solubility product constant (Ksp) of MoS2 (2.2 × 10− 56) and Bi2S3 (1.0 × 10− 97) [21], it is expected that Bi2S3 will preferentially deposit and form micro-flowers before MoS2. At the same time, the activation energy of the surface of the Bi2S3 micro-flowers would be weakened by the positively charged ions like Mo4+, which would provide high energy sites to promote the further growth of Bi2S3 [11, 21]. When Bi3+ is exhausted, the MoS2 nanosheets begin to grow in nucleation. Finally, the ultrathin MoS2 nanosheets are uniformly assembled onto the surface of the Bi2S3 micro-flowers to form MoS2/Bi2S3 heterojunction. The one-step hydrothermal method is easy to achieve a uniform and regular surface growth due to compatibility issues between MoS2 nanosheets and Bi2S3 micro-flowers, compared to the two-step method [21].
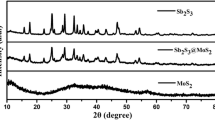
The crystal structures of the MoS2/Bi2S3 heterojunction are further characterized by XRD measurements. Figure 2 displays that the diffraction peaks of as-prepared Bi2S3 are corresponded well to that of the orthorhombic phase (JCPDS No. 17-0320) [22], while the peaks of MoS2 are identical to the standard diffraction data of MoS2 hexagonal phase (JCPDS No. 37-1492) [23]. All the MoS2/Bi2S3 heterojunctions synthesized with different loading amounts of MoS2 contain diffraction peaks corresponding to the Bi2S3, which prove the successful preparation of Bi2S3. But no intense characteristic diffraction peaks of MoS2 can be detected. This is related to only a few layers of MoS2 formed on the surface of Bi2S3, showing lower intensity than stacked MoS2 [5, 24, 25].
The SEM images of obtained Bi2S3, MoS2, and MoS2/Bi2S3-2 heterojunction are shown in Fig. 3. The pure Bi2S3 presents a typical rod-like morphologies, and some of them are self-assembled to form micro-flowers. The pure MoS2 sample shows the nanosheets agglomerated spherical structure (see Fig. 3b). It is observed that the MoS2/Bi2S3-2 heterojunction exhibits flower-like structure, in which Bi2S3 micro-flowers are wrapped with MoS2 nanosheets (see Figs. 3c and S1b). Further magnifying the heterojunction surface, it can be observed that the surface of Bi2S3 micro-flowers is evenly covered with ultrathin MoS2 nanosheets (see Fig. 3d). Furthermore, the SEM images of heterojunctions with different load of MoS2 are shown in Fig. S1. It is obvious that the load of MoS2 in the heterojunction increases with the increase of the dosage of (NH4)6Mo7O24·4H2O added to the reaction. As Fig. S1a shows, when a small amount of (NH4)6Mo7O24·4H2O is added, less MoS2 nanosheets appeared on the surface of the Bi2S3 micro-flowers. When an excessive dose of (NH4)6Mo7O24·4H2O is added, the formed MoS2 nanosheets become much thicker and much more, resulting in the partial agglomeration (See Fig. S1c). These results indicate the Bi2S3 micro-flowers play a role in the framework to avoid the agglomeration of the heterostructure [26]. But when the addition of (NH4)6Mo7O24·4H2O accumulates to a certain amount, the growth of a large amount of MoS2 nanosheets will still cause the agglomeration of heterojunction.
The detailed microstructure of MoS2/Bi2S3 heterojunction was further investigated by TEM. Figure 4a and b exhibits the typical TEM images of MoS2/Bi2S3-2, where it is clear that the nanorods of the Bi2S3 micro-flowers are coated with ultrathin MoS2 nanosheets. Figure 4c and d confirms the coexistence of MoS2 and Bi2S3 crystal lattices. The lattice fringes of 5.54 Å correspond to the (200) planes of Bi2S3 [27]. And the interlayer spacing of 6.03 Å belongs to (002) crystal plane of MoS2 [5, 28]. In addition, the formed intimate interfaces between Bi2S3 and MoS2 can be clearly observed, which indicate that the heterostructure was produced successfully. The observed intimated interfaces will benefit charge transfer between Bi2S3 and MoS2 [25]. Moreover, the thin MoS2 nanosheets grew on Bi2S3 with good dispersion endow the MoS2 with rich exposed edges and more active sites, which are expected to enhance the photocatalysis and electrochemical reaction activity of the heterojunction [24].
The chemical composition and states of constituent elements in MoS2/Bi2S3-2 heterojunction, pure MoS2, and Bi2S3 are investigated and compared by XPS measurement. As shown in the survey spectra in Fig. 5a, the diffraction peaks of Bi 5d, Bi 4f, Mo 3p, Mo 3d, and S 2p are observed, which indicates the composition of Bi, Mo, and S in the heterojunction. The extra O 1s and C 1s peak may be attributed to O2 and CO2 adsorbed from the texting environment [20, 26]. As shown in the high-resolution Bi 4f spectra, apart from the characteristic peaks at 162.2 and 161.1 eV belonging to S 2p1/2 and S 2p3/1, the binding energy peaks at 163.4 and 158.2 eV correspond to the characteristic peaks of Bi 4f2/5 and Bi 4f7/2, respectively, in the MoS2/Bi2S3-2 heterojunction (See Fig. 5b) [20, 29, 30]. Compared with pure Bi2S3, the binding energy of the as-fabricated heterojunction is not changed for the Bi 4f region. As observed in Fig. 5c, the binding energies located at 232.3 and 236.1 eV are derived from the Mo 3d3/2, and 228.9 eV is indexed to the Mo 3d5/2 in the pure MoS2. For MoS2/Bi2S3-2 heterojunction, Mo 3d3/2 (231.8 eV, 235.5 eV) and Mo 3d5/2 (228.4 eV) peaks shift to the lower binding energies compared to the pure MoS2, which can be attributed to the electronic interaction between Bi2S3 and MoS2 [31, 32]. The negative shift of binding energy indicates an increase of the electron density, suggesting that the photogenerated electrons will transfer from Bi2S3 to MoS2 [15, 33].
3.2 Photocatalytic properties
As discussed above, MoS2/Bi2S3 heterojunction exhibits an excellent combination of compositional and structural advantages, which is expected to improve photocatalytic activity. For the photocatalytic degradation of pollutants, both the adsorption capacity and the catalytic activity have a significant impact on efficient photocatalysis [11]. Figure 6a shows the photocatalytic activity of MoS2/Bi2S3 heterojunction, pure MoS2, and Bi2S3 under visible light for degradation MB. It is obvious that all heterojunctions present higher MB adsorption capacity than pure MoS2 or Bi2S3, because of the greatly enhanced surface area of the heterostructure (Figs. 6a and S2). However, the pure MoS2 and Bi2S3 have a severe aggregation structure (see Fig. 3), resulting in the low adsorption of MB. After considering the synergistic effect of adsorption and catalytic degradation, the overall MB removal efficiency of MoS2/Bi2S3-2 is maximum and considerably up to 76.2%, which is significantly higher than that of pure MoS2 (49.2%) and Bi2S3 (11.8%), as Fig. 6a shown. This is because MoS2/Bi2S3-2 based on its unique flower-like structure has a larger specific surface area to adsorb more MB dye. And the heterogeneous interfaces formed between Bi2S3 and MoS2 can effectively separate photogenerated electron–hole pairs to reduce their recombination rate, thereby greatly enhancing photocatalytic efficiency [34, 35]. The sample with few MoS2 layers (MoS2/Bi2S3-1) shows a reduced ability to degrade MB due to its relatively low active sites and increasing recombination chance of photogenerated electron–hole pairs, whereas the excessive MoS2 layers (MoS2/Bi2S3-3) due to its agglomeration also show a reduced ability to absorb and degrade MB.
a Variation of MB concentration with photodegradation time in the existence of various photocatalysts. b UV–Vis absorption spectra of MoS2/Bi2S3 heterojunction, MoS2, and Bi2S3. c Transient photocurrent responses of MoS2/Bi2S3 heterojunction, MoS2, and Bi2S3. d EIS Nyquist plots of MoS2/Bi2S3 heterojunction, MoS2, and Bi2S3
The UV–Vis absorption spectra of all photocatalysts are given in Fig. 6b. Both pure MoS2 and Bi2S3 show a broad absorption across the visible light spectral region. For the heterojunction, the growth of MoS2 nanosheets on the surface of Bi2S3 can benefit multiple light reflection and light harvest, which helps to enhance its absorption ability [21]. Therefore, MoS2/Bi2S3 heterojunction can be excited to generate more electron–hole pairs compared to pure MoS2 and Bi2S3 under visible light irradiation, which leads to an increase in its photocatalytic activity. To further understand the mechanism of the enhanced photocatalytic activity of MoS2/Bi2S3 heterojunction, the transient photocurrent responses of all samples are compared and shown in Fig. 6c. The higher photocurrent indicates the better electron and hole pairs separation efficiency, leading the higher photocatalytic activity [6, 36]. Obviously, all MoS2/Bi2S3 heterojunctions produce a higher photocurrent response than pure MoS2 and pure Bi2S3. In addition, the changing trend of transient photocurrent response is consistent with the photocatalytic evaluation. This is due to the formation of an intimate interface in heterojunction which is benefit to promote the charge transfer and improve the charge separation efficiency, thereby promoting the improvement of photocatalytic activity [37]. Among them, MoS2/Bi2S3-2 shows the highest photocurrent response compared with other samples, indicating that it has the highest photocatalytic performance, which has also been confirmed by photodegradation experiments. Furthermore, the EIS measurements are conducted to explore the charge separation and transfer processes (See Fig. 6d). As we all know, the charge transfer process at the corresponding electrode–electrolyte interface is greatly reflected from the arc radius of the Nyquist curve [38]. The smaller the arc radius, the lower the charge transfer resistance [34, 39]. Obviously, the arc diameter of MoS2/Bi2S3-2 is between pure Bi2S3 and pure MoS2, which indicates that the formation of the heterojunction would reduce the charge transfer resistance of the pure Bi2S3, promoting the rapid electron transport form the less conductive Bi2S3 to more conductive MoS2.
Above all, the photocatalytic mechanism of the MoS2/Bi2S3 heterojunction under visible light irradiation is initially proposed. The electronic band structures of pristine MoS2 and Bi2S3 are studied first. According to the Tauc formula, the band-gap energy of MoS2 and Bi2S3 are found to be ~ 1.14 and ~ 1.33 eV through the diffuse reflectance absorbance spectra, respectively (See Figs. S3 and S4). As Fig. S5 shows, the conduction band (CB) potential of MoS2 is estimated to be − 0.35 V vs. NHE and the CB potential of Bi2S3 is calculated to be − 0.59 V vs. NHE from the Mott-Schottky plots. The band position diagram of the heterojunction is proposed in Fig. 7, according to the attained results. Upon visible light irradiation, both MoS2 and Bi2S3 can be excited and generate the electron and hole pairs. Due to the matched energy band and intimate interfacial contact of MoS2 and Bi2S3, the excited electrons in Bi2S3 are injected into the CB of MoS2 while the photogenerated holes are transferred from the valence band (VB) of MoS2 to the Bi2S3, further realizing the suppression of the recombination of photogenerated electron–hole pairs [1, 13]. At the same time, the accumulated electrons in the CB of MoS2 reacted with the absorbed oxygen molecules on its surface to generate ·O2−, and the photogenerated hole in the VB of Bi2S3 can directly oxidize MB owing to its strong oxidation ability. Moreover, the standard redox potential of H2O/·OH (2.68 V vs. NHE) [40] is more positive than the valence band of Bi2S3, so that the Bi2S3 cannot oxidize H2O to form ·OH. The major proposed reaction steps during the photocatalytic process for the MoS2/Bi2S3 heterojunction are as follows:
3.3 Electrochemical properties
To study the electrochemical properties of MoS2/Bi2S3 heterojunction and pure Bi2S3, cyclic voltammetry (CV) and galvanostatic charge/discharge (GCD) tests are carried out. Figure 8a shows the CV curves of pure Bi2S3 and MoS2/Bi2S3 heterojunctions synthesized with different loading amounts of MoS2 at the scan rate of 20 mV/s in the voltage range of − 0.9 to − 0.3 V. It is obvious that the CV curves of all MoS2/Bi2S3 heterojunction electrodes exhibit approximately rectangular. Notably, compared to pure Bi2S3, all the MoS2/Bi2S3 electrodes exhibit a larger CV integrated area, which indicates the higher electrochemical activity of the MoS2/Bi2S3. The comparison of the GCD curves at the current density of 1A/g can also confirm the same phenomenon (in Fig. 8b). The discharging time of MoS2/Bi2S3 electrodes is all much longer than those of pure Bi2S3, further confirming the advantage of the heterostructure for supercapacitor performance improvement. The corresponding discharge capacitances of the MoS2/Bi2S3 electrodes are 64.2 F/g (MoS2/Bi2S3-1), 75.5 F/g (MoS2/Bi2S3-2), 100.2 F/g (MoS2/Bi2S3-3), and 79.7 F/g (MoS2/Bi2S3-5), which is larger than that of pure Bi2S3 (4.5F/g). Among them, MoS2/Bi2S3-3 exhibits the highest specific capacitance, compared to others.
In order to further evaluate the application potential of MoS2/Bi2S3-3 for supercapacitor, the CV curves and the GCD curves at different scan rates and different current densities are further studied, respectively. As Fig. 8c shows, the CV curves of MoS2/Bi2S3-3 exhibit a similar rectangle shape, and the shape is well maintained with the scan rate that increases from 10 to 40 mV/s, which suggests that MoS2/Bi2S3-3 has rapid interfacial kinetics and efficient electronic and ionic transport [24]. The GCD curves of the MoS2/Bi2S3-3 electrodes at different current densities are displayed in Fig. 8d. The curves are symmetric at all current densities, suggesting the high charge–discharge coulombic efficiency and low polarization of the electrodes [20]. Notably, both the CV curves and GCD curves of MoS2/Bi2S3-3 show superiority than pure Bi2S3 (Figs. S6a and b). To further explore the difference between MoS2/Bi2S3-3 and pure Bi2S3, the specific capacitances of these electrodes are calculated according to the GCD curves. As Fig. S7 shows, the MoS2/Bi2S3-3 electrodes exhibit specific capacitances of 111.7, 100.2, 87.0, and 80.3 F/g at 0.5, 1, 1.5, and 2 A/g, respectively, all of which are higher than that of pure Bi2S3 (10.1, 4.5, 3.76, and 2.56 F/g at 0.5, 1, 1.5, and 2 A/g). The above results demonstrate that MoS2/Bi2S3 heterojunction electrodes possess excellent electrochemical behavior. The reason comes down as follows: First, the unique structure of ultrathin MoS2 nanosheets on the surface of the Bi2S3 micro-flowers can provide large contact area between the electrode material and electrolyte, which can enhance the electrode material utilization and shorten the ion migration pathway during the charging-discharging process [6, 24]. Second, the high-quality heterointerface formed between MoS2 and Bi2S3 can provide an express path for electron transport [17, 41]. Moreover, the synergistic effects of the MoS2 and Bi2S3 further enhance the electrochemical activities in MoS2/Bi2S3 heterojunction. Therefore, the prepared MoS2/Bi2S3 heterojunction electrodes exhibit a high rate capability and an improved specific capacitance over the pure Bi2S3.
4 Conclusions
In summary, the 3D flower-like MoS2/Bi2S3 heterojunction by assembling ultrathin 2D MoS2 nanosheets onto 3D Bi2S3 micro-flowers has been successfully synthesized through a simple hydrothermal method. The special heterointerfaces and matched energy band between MoS2 and Bi2S3 can promote the charge spatial separation and inhibit the recombination of photogenerated electron–hole pairs, thus leading a much higher photocatalytic activity of MoS2/Bi2S3 heterojunction. The as-prepared MoS2/Bi2S3 heterojunction exhibits high photocatalytic activity for degrading MB with a 76.2% removal efficiency within visible light irradiation, which is significantly higher than that of pure MoS2 (49.2%) and Bi2S3 (11.8%). In addition, the unique heterointerfaces also can favor rapid electron conduction, which is beneficial for the enhancement of its electrochemical performance. The MoS2/Bi2S3 heterojunction possesses a higher specific capacitance of 100.2 F/g at a current density of 1 A/g, compared with pure Bi2S3. This work not only provides a facile method for the design and preparation of metal sulfides heterojunction but also provides a new idea for its multifunctional application.
References
S.V.P. Vattikuti, C. Byon, Superlattices Microstruct. 100, 514 (2016)
S. Kumar, S. Sharma, S. Sood, A. Umar, S.K. Kansal, Ceram. Int. 42, 17551 (2016)
Y. Zhong, Z. He, D. Chen, D. Hao, W. Hao, Appl. Surf. Sci. 467–468, 740 (2019)
K. Peng, J. Zhou, H. Gao, J. Wang, H. Wang, L. Su, P. Wan, ACS Appl. Mater. Interfaces 12, 19519 (2020)
Y. Yuan, P. Wang, Z. Li, Y. Wu, W. Bai, Y. Su, J. Guan, S. Wu, J. Zhong, Z. Yu, Z. Zou, Appl. Catal. B Environ. 242, 1 (2019)
S. Vadivel, A.N. Naveen, V.P. Kamalakannan, P. Cao, N. Balasubramanian, Appl. Surf. Sci. 351, 635 (2015)
V. Sabaghi, F. Davar, Z. Fereshteh, Ceram. Int. 44, 7545 (2018)
Z. Xu, H. Yan, W. Guopeng, M. Guijun, W. Fuyu, W. Lu, L. Can, J. Am. Chem. Soc. 130, 7176 (2008)
H. Shen, Z. Shao, Q. Zhao, M. Jin, C. Shen, M. Deng, G. Zhong, F. Huang, H. Zhu, F. Chen, Z. Luo, J. Colloid Interface Sci. 573, 115 (2020)
L. Ma, Q. Zhao, Q. Zhang, M. Ding, J. Huang, X. Liu, Y. Liu, X. Wu, X. Xu, RSC Adv. 4, 41636 (2014)
L. Long, J. Chen, X. Zhang, A. Zhang, Y. Huang, Q. Rong, H. Yu, NPG Asia Mater. 8, e263 (2016)
Q.A. Drmosh, A. Hezam, A.H.Y. Hendi, M. Qamar, Z.H. Yamani, K. Byrappa, Appl. Surf. Sci. 499, 143938 (2020)
J. Wang, J. Jin, X. Wang, S. Yang, Y. Zhao, Y. Wu, S. Dong, J. Sun, J. Sun, J. Colloid Interface Sci. 505, 805 (2017)
W. Bo, Z. Xin, Z. Nan, T. Zirong, X. Yijun, Langmuir 31, 4314 (2015)
L. Luo, X. Shen, L. Song, Y. Zhang, B. Zhu, J. Liu, Q. Chen, Z. Chen, L. Zhang, J. Alloys Compd. 779, 599 (2019)
E. Miniach, G. Gryglewicz, J. Mater. Sci. 53, 16511 (2018)
H. Liang, J. Lin, H. Jia, S. Chen, J. Qi, J. Cao, T. Lin, W. Fei, J. Feng, J. Power Sources 378, 248 (2018)
S.V.P. Vattikuti, A.K.R. Police, J. Shim, C. Byon, Sci. Rep. 8, 4194 (2018)
Y. Yang, H. Fei, G. Ruan, C. Xiang, J.M. Tour, Adv. Mater. 26, 8163 (2014)
Y. Ma, Y. Jia, L. Wang, M. Yang, Y. Bi, Y. Qi, J. Power Sources 342, 921 (2017)
C. Y, T. G, G. Q, CrystEngComm 17, 8720 (2015)
H. Zhang, L. Wang, Mater. Lett. 61, 1667 (2007)
D. Zhang, Q. Wang, Q. Wang, J. Sun, L. Xing, X. Xue, Electrochim. Acta 173, 476 (2015)
L. Fang, Y. Qiu, T. Zhai, F. Wang, M. Lan, K. Huang, Q. Jing, Colloid Surf. A 535, 41 (2017)
X. Qian, J. Zhang, Z. Guo, S. Liu, J. Liu, J. Lin, Appl. Surf. Sci. 481, 795 (2019)
J. Wang, C. Zhou, X. Yan, Q. Wang, D. Sha, J. Pan, X. Cheng, J. Mater. Sci. Mater. El. 30, 6633 (2019)
R. Xing, D. Li, C. An, L. Zhang, Q. Li, S. Liu, J. Nanosci. Nanotechnol. 12, 8029 (2012)
M. Alomar, Y. Liu, W. Chen, H. Fida, Appl. Surf. Sci. 480, 1078 (2019)
S. Wang, X. Li, Y. Chen, X. Cai, H. Yao, W. Gao, Y. Zheng, X. An, J. Shi, H. Chen, Adv. Mater. 27, 2775 (2015)
Z. Zhou, Y. Li, K. Lv, X. Wu, Q. Li, J. Luo, Mater. Sci. Semicond. Process. 75, 334 (2018)
H. Yu, Y. Xue, L. Hui, C. Zhang, Y. Li, Z. Zuo, Y. Zhao, Z. Li, Y. Li, Adv. Mater. 30, e1707082 (2018)
Y. Li, L. Huang, B. Li, X. Wang, Z. Zhou, J. Li, Z. Wei, ACS Nano 10, 8938 (2016)
B. Yang, K. Lv, Q. Li, J. Fan, M. Li, Appl. Surf. Sci. 495, 131–140 (2019)
S. Adhikari, D.-H. Kim, Appl. Surf. Sci. 511, 64 (2020)
Z. Gao, K. Chen, L. Wang, B. Bai, H. Liu, Q. Wang, Appl. Catal. B Environ. 268, 117–118 (2020)
H. She, P. Yue, J. Huang, L. Wang, Q. Wang, Chem. Eng. J. 392, 123813 (2020)
W. Jiang, X. Zong, L. An, S. Hua, X. Miao, S. Luan, Y. Wen, F.F. Tao, Z. Sun, ACS Catal. 8, 2209 (2018)
L. Zhang, C. Yang, K. Lv, Y. Lu, Q. Li, X. Wu, Y. Li, X. Li, J. Fan, M. Li, Chin. J. Catal. 40, 755 (2019)
S. Zhou, K. Chen, J. Huang, L. Wang, M. Zhang, B. Bai, H. Liu, Q. Wang, Appl. Catal. B Environ. 266, 118513 (2020)
P. Li, J. Li, X. Feng, J. Li, Y. Hao, J. Zhang, H. Wang, A. Yin, J. Zhou, X. Ma, B. Wang, Nat. Commun. 10, 2177 (2019)
S. Liu, S.C. Lee, U. Patil, I. Shackery, S. Kang, K. Zhang, J.H. Park, K.Y. Chung, S. Chan Jun, J. Mater. Chem. A 5, 1043 (2017)
Acknowledgements
This work is supported by the National Natural Science Foundations of China (Grant No.21978276), the Fundamental Research Funds for the Central Universities (Grant Nos. 2652019157; 2652019158; 2652019159), and Beijing Municipal Education Commission Key Science and Technology Project Fund (KZ201910853043).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest exists in the submission of this manuscript, and manuscript is approved by all authors for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liao, J., Zhong, Y., He, Z. et al. One-step synthesis of MoS2/Bi2S3 heterojunction with enhanced photocatalytic activity and high electrochemical performance. J Mater Sci: Mater Electron 32, 1022–1032 (2021). https://doi.org/10.1007/s10854-020-04877-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-04877-4