Abstract
The functional roles of freshwater mussels (Unionida) in tropical systems are poorly understood. We quantified the effects of mussel filtration, excretion and deposition in three anthropogenic tropical systems, i.e. a man-made lake, abandoned mining pool and rice paddy channel. Sinanodonta cf. woodiana (non-native) was present at all three sites, whilst Pilsbryoconcha compressa (native) was present in the channel only. Clearance rates, biodeposition rates and effects on suspended algal pigment and dissolved nutrient concentrations were quantified in controlled, replicated experiments in laboratory tanks with water from original habitats. Clearance rates were generally low and did not explain the high biodeposition rates observed. A considerable proportion of the natural diet of these populations may therefore consist of material that was not available in tanks, i.e. benthic or deposited algae. Deposition rates in lake and channel populations exceeded published rates from temperate and Mediterranean habitats, presumably due to prevalence of non-palatable material and/or higher metabolic rates in tropical systems. The presence of S. cf. woodiana but not P. compressa led to a strong increase in total ammonia nitrogen concentrations and N:P ratios, exceeding estimations from other systems. This study suggests that freshwater mussels play different functional roles in anthropogenic tropical habitats than in temperate systems.
Similar content being viewed by others
Introduction
Freshwater mussels (Unionida) play key functional roles in lotic and lentic ecosystems around the globe, including filter feeding, nutrient cycling and biodeposition (Vaughn & Hakenkamp, 2001). Mussels are generally considered to be suspension feeders, feeding on phytoplankton, bacteria and other suspended material, excreting nutrients (predominantly ammonia nitrogen TAN (= NH3 and NH4) and phosphate PO4) to the water column, and depositing organic matter to the sediment as non-assimilated pseudofaeces and egested faeces (Zieritz et al., 2019 and references therein). At high population densities, which can commonly reach > 100 and in some cases > 1000 individuals/m2 (Ostrovsky & Popov, 2011; Chowdhury et al., 2016) or up to 90% of the benthic biomass (Negus, 1966; Layzer et al., 1993; Parmalee & Bogan, 1998), mussels can have profound effects on aquatic ecosystems. Freshwater mussel filter feeding can control phytoplankton abundance (Welker & Walz, 1998; Chowdhury et al., 2016), nutrient excretion can alleviate nutrient limitation, alter phytoplankton communities and improve water quality (Atkinson et al., 2013), and biodeposition of nutrients and organic material can lead to increases in benthic biodiversity and abundance (Spooner & Vaughn, 2006, 2012; Chowdhury et al., 2016). The rates and impacts of these processes on the ecosystem vary with population density, species and size composition of the mussel community, and environmental conditions, such as discharge, temperature and food availability (Vaughn, 2018).
Over recent decades, a substantial body of research has improved understanding of the functional roles performed by freshwater mussels (reviewed by Vaughn & Hakenkamp, 2001; Vaughn, 2018). This knowledge is important given the drastic declines in freshwater mussel diversity and population sizes, as ecosystem functions are lost with the loss of populations. In North America, 11% of species are already extinct, and declines in population sizes and ranges of other species commonly exceed 80% (IUCN, 2019). Although much less well documented, declines are likely similar or higher in tropical Southeast Asia, where freshwater mussel diversity and endemism are particularly high, and anthropogenic threats are severe and numerous (Zieritz et al., 2018b).
Freshwater habitats in the tropics differ to those in temperate and Mediterranean systems in terms of annual temperature and discharge regimes, nutrient cycling and limitation, and food availability (Boulton et al., 2008; Zieritz et al., 2019 and references therein). Tropical anthropogenic freshwater habitats, such as reservoirs, ponds and rice paddy streams, are particularly common and provide considerable value to humans by facilitating recreation, fishing, aqua- and agriculture and other activities (Yusoff et al., 2006). These systems are generally nutrient-rich and often hypereutrophic (Cunha et al., 2013; Walsh et al., 2014). In Malaysia, anthropogenic freshwater habitats are predominantly and often densely inhabited by the non-native invasive Sinanodonta cf. woodiana (Lea, 1834), an introduction from the Yangtze River basin (Lopes-Lima et al., 2020), whilst native species are comparatively rare and may be outcompeted by S. cf. woodiana (Zieritz et al., 2016, 2018b, c).
Despite the ubiquity of freshwater mussels in tropical anthropogenic habitats, their functional roles in these systems are poorly understood. To our knowledge, quantified filtration and nutrient excretion rates are restricted to a single species in a single tropical anthropogenic system. In a mesocosm and parallel laboratory experiment, Zieritz et al. (2019) revealed considerable differences in the role of mussels in a man-made lake compared to a natural, mesotrophic stream in tropical Malaysia. Net rates of change in TAN concentrations in tanks with mussels compared to control tanks averaged + 51 µg N mussel−1 h−1 for S. cf. woodiana from the lake but only + 3 µg N mussel−1 h−1 for the native species (Contradens contradens (Lea, 1838) and Monodontina vondembuschiana (Lea, 1840)) from the stream. On the other hand, chlorophyll a (chl a) clearance rates of mussels from the stream environment appeared to exceed those by lake mussels. However, the extent to which these differences were caused by differences in seston quantity and quality between the habitats, by a species effect, or a combination of the two, is not known. In addition, the design of that study did not allow authors to distinguish between deposited and un-ingested material; thus, deposition rates of tropical mussels remain unquantified. Considering the predominance and predicted future spread of S. cf. woodiana and simultaneous decline of native species across the region (Gallardo et al., 2018), a better understanding of the effects of S. cf. woodiana and sympatric native species on their environment seems particularly warranted.
The present study aims to quantify and compare the effects of filtration, excretion and deposition of native and non-native freshwater mussels in anthropogenic tropical systems. Data on clearance rates, deposition rates and effects of mussels on suspended pigment and dissolved nutrient concentrations were collected from three typical anthropogenic tropical habitats under semi-natural conditions (i.e. in the laboratory in untreated water from the original habitat). Data were then compared with published data on mussel populations from temperate, Mediterranean and subtropical regions.
Methods
Study sites
The study was conducted on four mussel populations and water from three typical anthropogenic freshwater habitats that are commonly inhabited by freshwater mussels in the tropics (Zieritz et al., 2016, 2018c), i.e. a man-made lake, an abandoned mining pool and a rice paddy channel.
-
(1)
Semenyih Lake (hereafter “Lake”) within the Langat River catchment is a man-made water body located in a recreational zone about 3 km from Semenyih, a town with a population of approximately 90,000 (Table 1). The lake has a circumference of approximately 2.3 km, a surface area of around 60,000 m2 and reaches a maximum depth of 2 m; pH is 6.5–7 (Zieritz et al., 2016). No macrophytes were visible at the time of sampling, and the substrate was predominantly mud, with sand and silt at some locations (pers. obs.). The only mussel present in the lake is Sinanodonta cf. woodiana (Sw), which occurs at moderate to high densities averaging about 13–50 individuals m−2 (Zieritz et al., 2018a, 2019). In July 2015 (dry season), the lake was classified as eutrophic and N-limited (Table 1, Zieritz et al., 2019).
Table 1 Geographical coordinates, mussel species richness and water quality parameters at the three study sites -
(2)
The second site is an abandoned mining pool in the former tin mining area of Bestari Jaya (“Pool”), which covers an area of 360 ha and is part of the River Selangor catchment (Ashraf et al., 2012). The pool has a circumference of around 600 m, is 16,000 m2 in surface area and 1.5 m in depth, with a pH of around 6 and chloride concentrations of about 1.5 mg/l (Ashraf et al., 2012). Substrate is predominantly mud, and water hyacinths (Eichhornia crassipes (Mart.) Solms)) were prevalent at time of sampling (pers. obs.). Sw is the only freshwater mussel inhabiting the mining pool, attaining average densities of > 50 individuals m−2 (Zieritz et al., 2018a).
-
(3)
The rice paddy channel (site “Channel”) lies within the River Perak catchment, and is situated near the village of Kampung Gajah. It is narrow (averaging 1.5 m width) and shallow (< 50 cm depth), with a pH of about 6.3 and specific conductivity of 98 µS/cm (Zieritz et al., 2016). No macrophytes were visible at time of sampling, and substrate was predominantly mud with some sand (pers. obs.). The channel is inhabited by two freshwater mussel species, the native Pilsbryoconcha compressa (Martens, 1860) (Pc) and Sw, which are present at low to moderate densities (< 20 individuals m−2) (pers. obs.; Zieritz et al., 2018a).
Experimental setup
This controlled, replicated experiment was designed to assess the effects of mussel feeding, excretion and biodeposition on the water column and benthos under semi-natural conditions. Experiments were carried out under natural light conditions at 25°C air temperature at the University of Nottingham Malaysia in the wet season on 25 Oct (Lake), 16 Nov (Channel) and 22 Nov 2017 (Pool). Air temperatures at the study sites in the study months ranged from 23 to 32°C (https://www.timeanddate.com/). In the afternoon of the day before the experiments, a total of 15 (for Sw from Lake and Pool, respectively), 11 (for Pc from Channel) and 9 (for Sw from Channel) mussels were randomly sampled from each site following the systematic sampling design of Strayer and Smith (2003). Three random starting points were determined by throwing a stone in the water from the bank at 50-m distance from each other. Additional sampling points per starting point were placed at 10-m intervals. At each sampling point, the first mussel encountered was sampled. In addition, approximately 120 l water was taken from each site, and mussels and water transported to the laboratory in darkened containers. Mussels were kept overnight at 25°C air temperature in water of their respective site with aeration to minimise disturbance.
The morning after sampling, about 16 h after mussels had been collected from their habitats, 20 (for Lake and Pool) and 25 (for Channel) transparent, cylindrical, 3.6 l plastic tanks were set up. Each tank was equipped with an air stone connected to an air pump and filled with 3.5 l of water from the respective site that was previously thoroughly homogenised through stirring in a 25 l water tank, which was continuously filled up with new source water. To provide a medium for biodeposit analysis, 60 g of sediment in the fine to coarse sand size fractions (0.212–1 mm) was added to the bed of each tank. Prior to use, this sediment was sieved (to ensure known and consistent sizes for each tank), burned in a furnace at 550°C for 3 h to remove organic material and homogenised by stirring. Sediment was allowed to settle completely for 20 min after being added to tanks. After that, a 0.5 l water sample was taken from each tank for analysis of initial pigment, nutrient and organic matter (OM) concentrations, leaving 3 l of water in each tank. The shell of each mussel was carefully cleaned underwater with a soft brush to remove epibionts and other material, and a single mussel was placed on a net submerged at 50% water depth inside each tank with the exception of 5 randomly selected tanks, which served as controls. After 3 h, mussels were removed from the tanks, and water was carefully pumped into clean containers for determination of final pigment and nutrient concentrations (see below); to avoid disturbing the sediment and deposits not all water were pumped out (3 cm was left in each tank). All sediments were collected into sealable bags using a spoon for determination of concentrations of settled/deposited pigment and OM. Mussel soft tissue was dissected from each specimen, dried at 80°C for 48 h and dry weight (DW) measured to ± 0.01 g accuracy.
Effects on the water column
Water samples were refrigerated and processed as soon as possible on the day of the experiment. A known volume of each water sample (0.05–0.2 l, depending on turbidity) was filtered through Whatman® GF/C-filters with a vacuum pump. Filters were frozen, and filtered and unfiltered water samples were kept cool for subsequent determination of a number of parameters. Concentrations of chlorophyll a (chl asuspended) and carotenoids (carotenoidssuspended) in water were assessed spectrophotometrically (Mackereth et al., 1989). As carotenoids are more likely to pass through invertebrate guts undigested than chl a (McLeroy-Etheridge & McManus, 1999), an increase in carotenoid:chl a ratio in mussel tanks would indicate that algal material is digested by mussels rather than simply ejected as undigested pseudofaeces. Pigments were extracted from filters by soaking them in 5 ml extraction solvent (acetone, methanol and distilled H2O in a ratio of 80:15:5; Leavitt & Hodgson, 2001) for 24 h at 4°C in the dark. After that, the pigment solution was transferred to centrifuge tubes, another 5 ml extraction solvent added, centrifuged for 10 min, and the supernatant analysed spectrophometrically by trichromatic pigment analysis following Jeffrey and Humphrey (1975) and Strickland and Parsons (1972). Concentrations of soluble reactive phosphorus (SRP) and total ammonia nitrogen (TAN) were determined from filtered water samples using the ascorbic acid and phenate reduction methods, respectively (Mackereth et al., 1989).
In addition, organic matter content (OM) and total phosphorus (TP) concentrations were determined for initial water samples to obtain data on baseline conditions. OM concentration was determined by loss-on-ignition analysis of a frozen filter, with an initial drying step at 103°C for 24 h and subsequent ignition at 550°C for 4 h. Dried and ignited filter weight was measured to ± 0.001 g accuracy. For TP analysis, 1.5 ml sulphuric acid were immediately added to a 25 ml unfiltered water sample, which was subsequently analysed by ascorbic acid method (Mackereth et al., 1989).
Effects on the sediment
Deposited material was analysed from sediments. For this purpose, sediments were frozen immediately after sampling and later freeze-dried on an Alpha 1-2LDplus (CHRiST, Germany) at − 40°C and 0.12 atm for 72 h. Concentrations of chlorophyll a (chl adeposited) and carotenoidsdeposited were determined for each tank on 5 g subsamples using the same methods as above. In addition, organic matter content (OMdeposited) was determined on 5 g subsamples by loss-on-ignition analysis at 550°C for 4 h. Concentrations in 5 g subsamples were then converted to biodeposition rates [h−1 mussel DW−1].
Data analysis
Differences in baseline conditions between the three study sites in terms of pigment, nutrient and organic matter concentrations were assessed based on initial concentrations in replicate tanks using ANOVA (Table 1).
Rates of change in suspended pigment and dissolved nutrient concentrations in the water column [h−1] were determined for each replicate tank using the formula (Ct – C0) × V/T, where Ct and C0 are final and initial nutrient or pigment concentrations, respectively, V is the volume of water in the tank (3 l) and T is incubation time (3 h). Net rates of change in suspended pigment and dissolved nutrient concentration, and deposition rates (e.g. net chl asuspended) were calculated by deducting average values observed in control tanks from respective mussel tanks. Clearance rates [l h−1] of chl a (CRchl a) were calculated for each tank using the formula ln (C0/Ct) × V/T (following Cyr et al. (2017)), and deducting respective rates obtained in control tanks. The measurement error (s) for each parameter was calculated as the median of standard deviations of randomly taken triplicates of measurements of initial concentrations, which were ln transformed for clearance rates. The lowest detectable clearance and net rates (RateDL) for each parameter were then calculated as RateDL = 1.96 s × V/T.
Whether mussels had a significant effect on suspended pigment, dissolved nutrient, as well as deposited pigment and OM concentrations was tested in two ways. Firstly, Welch Two-Sample t tests (also known as unequal variances t test) were used to compare rates of change in mussel and control tanks on each of the four study populations. Secondly, one-sample t tests or Wilcoxon-Signed Rank tests (for non-normal data) were conducted on each of the four study populations to test whether net rates of change, deposition and clearance rates were significantly different from 0. Tests were run as one-sided tests for chl adeposited, carotenoidsdeposited and OMdeposited.
Linear regressions and ANCOVAs (including “population” as a factor with four levels) were used to test for the effect of mussel size (tissue dry weight, DW; covariate) on clearance and deposition rates, and net rates of change in suspended pigment and dissolved nutrient concentrations. Clearance and net rates below RateDL as well as outlier values were excluded from analyses. Datasets that retained < 5 data points per population were excluded from ANCOVAs. Data were tested for normality and homogeneity of variance (for ANCOVAs) and, if necessary, transformed prior to analyses.
Results
Comparison of study sites and general observations
The three study sites differed significantly in water quality parameters and other characteristics (Table 1). Concentrations of nutrients and algal pigments in the water in November 2017 were significantly higher in the Channel and Pool, both classified as hypereutrophic, compared to the Lake, which was classified as mesotrophic. Molar DIN:DIP ratios ranged from 35:1 to 235:1 (Table 1).
Mussels were observed feeding (as indicated by open siphons), excreting and depositing (pseudo) faeces during both the 16-h acclimatisation period in holding tanks and 3-h experimental period in experimental tanks.
Calculated detection limits were CRchla-DL = 0.29 l h−1, net chlasuspended-DL = 9.11 µg h−1, net carotenoidssuspended-DL = 9.60 µg h−1, net TANDL = 21.21 µg h−1, net SRPDL = 3.67 µg h−1, net chladeposited-DL = 0.05 µg h−1, net carotenoidsdeposited-DL = 0.15 µg h−1 and net OMdeposited-DL = 0.40 mg h−1.
Clearance rates and changes to algal pigment concentrations in the water column
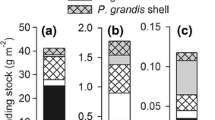
None of the four mussel populations significantly affected suspended chl a concentrations (P > 0.05 in Welch Two-Sample t tests on chl asuspended of mussel vs. control tanks; Fig. 1a; P > 0.05 in t tests on net chl asuspended of mussel vs. control tanks; Table 2). However, chl a clearance rate was significantly above 0 in the Lake population (one-sample t test: t = 2.171, df = 14, P = 0.0476), averaging 0.6 l−1 mussel−1 h−1 (Table 2). In addition, the two Channel populations caused a significantly positive net increase in suspended carotenoid concentrations (one-sample t tests: Sw-Channel: t = 2.698, df = 8, P = 0.0272; Pc-Channel: t = 3.2156, df = 10, P = 0.0092). On average, mussels greatly increased the ratio of suspended carotenoid:chl a ratios compared to background ratios (Table 2).
Boxplots of change in a, b suspended pigment, c, d dissolved nutrients and e–h deposited material after 3 h in 3 l of water from Semenyih Lake (Lake), an abandoned mining pool (Pool) and a rice paddy channel (Channel) with single mussels (Pc, Pilsbryoconcha compressa; Sw, Sinanodonta cf. woodiana) collected from the respective sites and without mussels (C). Numbers in brackets indicate numbers of replicates. Box limits represent third and first quartile; centre horizontal line represents median; vertical lines represent maximum and minimum; dots represent outliers. Asterisks indicate significant differences between control and mussel tanks within each site determined by Welch Two-Sample t tests (*P < 0.05, **P < 0.01, ***P < 0.001). chl a chlorophyll a, OM organic matter, SRP soluble reactive phosphorus (PO4–P), TAN total ammonia nitrogen (NH4–N + NH3–N)
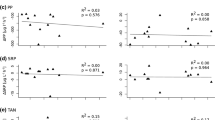
Mussel size (DW) did not significantly affect CRchla and net carotenoidssuspended (effect on net chl asuspended could not be tested due to low replicate number per population retained after removal of values < RateDL and outliers; Fig. 2a and g; Table 3).
Relationship between mussel size (dry weight) and a clearance rates, and net (i.e. after deduction of average values observed in control tanks) b–d deposition rates, and net rates of change in e, f dissolved nutrient and g suspended carotenoid concentrations in the water column for four mussel populations in laboratory tank experiments. Values below the detection limit as well as outlier values not shown. Only regression lines significant at the 0.05 level are shown (full lines, across all four populations; dashed lines, S. woodiana—Channel). chl a chlorophyll a, OM organic matter, SRP soluble reactive phosphorus (PO4–P), TAN total ammonia nitrogen (NH4–N + NH3–N)
Biodeposition
Settling seston was visible to the naked eye in both control and mussel tanks, as was biodeposition of (pseudo)faeces in mussel tanks. Biodeposition of chl a by mussels was significantly different between control and mussel tanks in all study populations with the exception of Sw-Channel; this site, however, showed statistically significant biodeposition of carotenoids and OM (Fig. 1e–g). Biodeposition rates were particularly high in the Sw-Lake population, and lowest and least pronounced in the Sw-Pool population (Table 2). Deposition rates consistently exceeded removal rates of pigments in all four populations (Table 2). When compared to average background ratios of suspended carotenoids:chl a ratios in their respective habitats (Table 1), Lake mussels deposited about twice as much carotenoid compared to chl a, whilst the ratio remained constant or even decreased in the other three populations (Table 2).
Mussel size (DW) was significantly positively correlated with deposition rates of chl a, but not carotenoids and OM, across the whole dataset (Figs. 2b–d, Table 3). However, the effect of mussel size on net rates of change in chl adeposited was not consistent in the four study populations (as indicated by a significant effect of the interaction factor). A significant, positive correlation between mussel size and net rates of change in chl adeposited was found only in the Sw-Channel population (Fig. 2b; Linear regression: F1,6 = 20.85, P = 0.004).
Dissolved nutrients
The presence of mussels in tanks led to an increase in TAN in Lake and Pool populations, but this effect was not statistically significant in the Channel populations, where TAN concentrations in control tanks on average increased by 90 µg h−1 and rates of change in TAN concentrations in several mussel tanks were below this background rate (Fig. 1c, Table 2). On average, Sw increased TAN in tanks at a rate of 52–197 µg N h−1 mussel−1 (Table 2). Net rate of change in TAN concentration was significantly positively correlated with mussel size across the whole dataset (Table 3, Fig. 2e). A significant, positive correlation between mussel size and net rates of change in TAN concentration was also found in the Sw-Channel population (Fig. 2e; Linear regression: F1,5 = 13.53, P = 0.014).
Mussel presence did not significantly affect SRP concentrations in any population (Fig. 1d), but net rates of change in SRP concentration were significantly below 0 in the Sw-Lake and significantly above 0 in the Pc-Channel population (Table 2). Across the whole dataset, net rates of change in SRP concentration significantly decreased with mussel size (Table 3), but no statistically significant correlation between mussel size and net rates of change in SRP concentrations was found within any of the four study populations (Fig. 2f).
Discussion
Filtration
Average clearance rates of freshwater mussel populations from temperate habitats commonly exceed 0.5 l mussel−1 h−1 (Kryger & Riisgård, 1988; McIvor, 2004; Cyr et al., 2017) and up to > 10 l mussel−1 h−1 under continuous supply of algae (Byllaardt & Ackerman, 2014; Douda & Čadková, 2018). In comparison, clearance rates measured here in populations from three anthropogenic tropical habitats were low, averaging only 0.04–0.6 l mussel−1 h−1, and not statistically significantly different from 0 with exception of the S. cf. woodiana Lake population. In a number of tanks, especially those with mussels from the two rice paddy channel populations and small individuals, mussel presence actually led to an increase in suspended pigment concentrations. In addition, mussel presence in the rice paddy channel tanks on average led to a net increase in suspended carotenoid concentrations, and deposition rates exceeded removal rates of pigments in all four populations (see further discussion on this below). In combination, these observations suggest that mussels fed at a lower than natural rate, depositing and ejecting algae (including material ingested before the start of the experiment) at a faster rate than taking them up.
This result was contradictory to a number of previous studies using a similar experimental setup (i.e. estimating clearance rate on non-starved mussels), which generally reported positive clearance rates across replicate mussel tanks (Vanderploeg et al., 1995; Chowdhury et al., 2016; Cyr et al., 2017; Douda & Čadková, 2018; Zieritz et al., 2019), although negative clearance rates were observed in some populations but omitted from the analysis (e.g. 4 out of 8 replicates from Tarawera Lake, New Zealand; Cyr et al., 2017). Another difference to previous studies was the lack of a significant relationship between mussel size and clearance rates. In filter-feeding bivalves, this relationship is usually strongly positive, as pumping (filtration) rate increases with gill area (Cranford et al., 2011).
There are several possible explanations for these unexpected results. Firstly, filtration rates may have been lower than those in the natural system due to a decrease in the quantity of high-quality, palatable phytoplankton available in the tanks during the experiment. Additionally or alternatively, a considerable proportion of the mussels’ natural diet may have consisted of algal material that was not available in the tanks, i.e. benthic or deposited algae. Although freshwater mussels are generally considered to be suspension feeders, growing evidence indicates that deposit feeding can contribute to 40–80% of their diet (Raikow & Hamilton, 2001; Collier et al., 2017; Weber et al., 2017). Deposit feeding in freshwater mussels is largely unexplored but may be particularly important in environments where the proportion of unpalatable, e.g. filamentous (Nichols & Garling, 2000), phytoplankton is high, rendering suspension feeding energetically inefficient.
Biodeposition
Compared to previously published data (Vaughn et al., 2004; Howard & Cuffey, 2006; Collier et al., 2017), biodeposition rates for our study populations were moderate (for chl a) and insignificant (for OM) for S. cf. woodiana from the abandoned mining pool, but 3–30 times higher for all other populations. Deposition rates presented by Vaughn et al. (2004) and Collier et al. (2017) may potentially be considered underestimations as they are based on mussels that had been starved and thus exclude already ingested material, and were subsequently fed cultured algae rather than their natural diet during. However, Howard and Cuffey’s (2006) experimental setup on Margaritifera falcata (Gould, 1850) from the South Fork Eel River, California, was similar to ours and did not involve a starving period. Compared to the rates of approximately 3–4 mg OM mussel−1 h−1 observed by Howard and Cuffey (2006), we attribute the 3-4 times higher deposition rates observed in three of our four study populations to the prevalence of non-palatable material and/or higher metabolic rates at higher temperatures in tropical anthropogenic study systems (Gillooly et al., 2001). We further argue that mussel deposition may therefore be particularly important to benthic communities in certain tropical freshwater systems. As in the present experiments, Howard and Cuffey (2006) did not observe a statistically significant relationship between biodeposition of OM and size of M. falcata.
Excretion
Average net rates of change in TAN concentrations of S. cf. woodiana populations from the mesotrophic lake were similar to those obtained by us from the same site in July 2015 (Table 1; Zieritz et al., 2019) and comparable to previously published N-excretion rates, which typically range around 10–70 µg TAN mussel−1 h−1 (Nalepa et al., 1991; Vaughn et al., 2004; Cyr et al., 2017). However, respective rates by the S. cf. woodiana population from the hypertrophic mining pool were at least 2–3 times that of previously reported values, reaching maximum individual rates of > 400 µg TAN mussel−1 h−1. Contrary to most previous studies (Nalepa et al., 1991; Vaughn et al., 2004; Cyr et al., 2017; Zieritz et al., 2019), the relationship between mussel size and N-excretion rate was statistically significant only in one of the four populations.
The effect of mussel presence on ambient SRP-concentrations, on the other hand, was generally weak. Net rates of change in SRP concentrations of the three S. cf. woodiana study populations were either close to 0 or, in the Lake population, negative, thereby confirming results by Zieritz et al. (2019) on the same population in July 2015 (Table 1). This non-native species therefore appears to have a particularly high demand for P (Christian et al., 2008) and heavily increases ambient N:P ratios at levels previously unseen in freshwater mussels (Nalepa et al., 1991; Vaughn et al., 2004; Atkinson et al., 2013; Cyr et al., 2017). P. compressa, on the other hand, significantly increased ambient SRP-concentrations and appeared to excrete at a ratio comparable with previously published data, thereby potentially alleviating the apparent P-limitation of primary production of the rice paddy channel, where DIN:DIP ratio was about 15 times higher than Redfield ratio.
Effects of mussels in anthropogenic tropical freshwater habitats
Our results indicate that filter feeding of mussels in certain anthropogenic tropical freshwater habitats, particularly hypertrophic ones, may occur at considerably lower rates than in temperate and Mediterranean systems. Whilst mussels therefore do not appear to play a significant role in terms of increasing water clarity in these systems, they may strongly affect their ecosystems through high rates of N-excretion and deposition of organic material. The non-native S. cf. woodiana increased ambient TAN concentrations and TAN:SRP ratios at rates far exceeding those reported from other Unionidae populations. In contrast, these rates were considerably lower and comparable to previously published values in the native P. compressa population, sympatrically living with S. cf. woodiana in the rice paddy channel. This suggests the possibility of antagonistic effects of different species on nutrient cycling and limitation. High biodeposition rates, such as those observed in the lake and channel populations of this study, suggest that mussels may strongly affect benthic communities in these systems. However, the importance of deposit feeding for tropical freshwater mussel populations and the extent to which their biodeposits are derived from deposit feeding and subsequently recycled to the benthos remain to be quantified.
References
Ashraf, M. A., M. J. Maah & I. Yusoff, 2012. Morphology, geology and water quality assessment of former tin mining catchment. The Scientific World Journal 2012: 369206.
Atkinson, C. L., C. C. Vaughn, K. J. Forshay & J. T. Cooper, 2013. Aggregated filter-feeding consumers alter nutrient limitation: consequences for ecosystem and community dynamics. Ecology 94: 1359–1369.
Boulton, A. J., L. Boyero, A. P. Covich, M. Dobson, S. Lake & R. Pearson, 2008. Are tropical streams ecologically different from temperate streams? In Dudgeon, D. (ed.), Tropical Stream Ecology. Elsevier, Amsterdam: 257–284.
Byllaardt, J. & J. D. Ackerman, 2014. Hydrodynamic habitat influences suspension feeding by unionid mussels in freshwater ecosystems. Freshwater Biology 59: 1187–1196.
Chowdhury, G. W., A. Zieritz & D. C. Aldridge, 2016. Ecosystem engineering by mussels supports biodiversity and water clarity in a heavily polluted lake in Dhaka, Bangladesh. Freshwater Science 35: 188–199.
Christian, A. D., B. G. Crump & D. J. Berg, 2008. Nutrient release and ecological stoichiometry of freshwater mussels (Mollusca:Unionidae) in 2 small, regionally distinct streams. Journal of the North American Benthological Society 27: 440–450.
Collier, K. J., S. J. Clearwater, P. H. M. W. Neijenhuis & S. A. Wood, 2017. Factors influencing biodeposit production by the New Zealand freshwater mussel Echyridella menziesii. New Zealand Journal of Marine and Freshwater Research. https://doi.org/10.1080/00288330.2017.1307234.
Cranford, P. J., J. E. Ward & S. E. Shumway, 2011. Bivalve filter feeding: variability and limits of the aquaculture biofilter. In Shellfish Aquaculture and the Environment :81–124.
Cunha, D. G. F., M. D. C. Calijuri & M. C. Lamparelli, 2013. A trophic state index for tropical/subtropical reservoirs (TSItsr). Ecological Engineering 60: 126–134.
Cyr, H., K. J. Collier, S. J. Clearwater, B. J. Hicks & S. D. Stewart, 2017. Feeding and nutrient excretion of the New Zealand freshwater mussel Echyridella menziesii (Hyriidae, Unionida): implications for nearshore nutrient budgets in lakes and reservoirs. Aquatic Sciences 79: 557–571.
Douda, K. & Z. Čadková, 2018. Water clearance efficiency indicates potential filter-feeding interactions between invasive Sinanodonta woodiana and native freshwater mussels. Biological Invasions 20: 1093–1098.
Gallardo, B., A. E. Bogan, S. Harun, L. Jainih, M. Lopes-Lima, M. Pizarro, K. A. Rahim, R. Sousa, S. G. P. Virdis & A. Zieritz, 2018. Current and future effects of global change on a hotspot’s freshwater diversity. Science of the Total Environment 635: 750–760.
Gillooly, J. F., J. H. Brown, G. B. West, V. M. Savage & E. L. Charnov, 2001. Effects of size and temperature on metabolic rate. Science 293: 2248–2251.
Howard, J. K. & K. M. Cuffey, 2006. The functional role of native freshwater mussels in the fluvial benthic environment. Freshwater Biology 51: 460–474.
IUCN, 2019. The IUCN Red List of Threatened Species. Version 2018-2. In. http://www.iucnredlist.org.
Jeffrey, S. W. & G. F. Humphrey, 1975. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochemie und Physiologie der Pflanzen 167(2): 191–194.
Kryger, J. & H. U. Riisgård, 1988. Filtration rate capacities in 6 species of European freshwater bivalves. Oecologia 77: 34–38.
Layzer, J. B., M. E. Gordon & R. M. Anderson, 1993. Mussels: the forgotten fauna of regulated rivers: a case study of the Caney Fork River. Regulated Rivers: Research and Management 8: 63–71.
Leavitt, P. R. & D. A. Hodgson, 2001. Sedimentary Pigments. In Smol, J. P., H. J. B. Birks & W. M. Last (eds), Tracking Environmental Change Using Lake Sediments Volume 3: Terrestrial, Algal, and Siliceous Indicators. Kluwer Academic Publishers, Dordrecht.
Lopes-Lima, M., A. Hattori, T. Kondo, J. Hee Lee, S. Ki Kim, A. Shirai, H. Hayashi, T. Usui, K. Sakuma, T. Toriya, Y. Sunamura, H. Ishikawa, N. Hoshino, Y. Kusano, H. Kumaki, Y. Utsugi, S. Yabe, Y. Yoshinari, H. Hiruma, A. Tanaka, K. Sao, T. Ueda, I. Sano, J.-I. Miyazaki, D. V. Gonçalves, O. K. Klishko, E. S. Konopleva, I. V. Vikhrev, A. V. Kondakov, M Yu Gofarov, I. N. Bolotov, E. M. Sayenko, M. Soroka, A. Zieritz, A. E. Bogan & E. Froufe, 2020. Freshwater mussels (Bivalvia: Unionidae) from the rising sun (Far East Asia): phylogeny, systematics, and distribution. Molecular Phylogenetics and Evolution 146: 106755.
Mackereth, F. J. H., J. Heron & J. F. Talling, 1989. Water Analysis: Some Revised Methods for Limnologists. Freshwater Biological Association Special Publication, Ambleside.
McIvor, A., 2004. Freshwater mussels as biofilters. PhD thesis, University of Cambridge.
McLeroy-Etheridge, S. L. & G. B. McManus, 1999. Food type and concentration affect chlorophyll and carotenoid destruction during copepod feeding. Limnology & Oceanography 44: 2005–2011.
Nalepa, T. F., W. S. Gardner & J. M. Malczyk, 1991. Phosphorus cycling by mussels (Unionidae: Bivalvia) in Lake St Clair. Hydrobiologia 219: 230–250.
Negus, C. L., 1966. A quantitative study of growth and reproduction of unionid mussels in the River Thames at Reading. Journal of Animal Ecology 35: 513–532.
Nichols, S. & D. Garling, 2000. Food-web dynamics and trophic-level interactions in a multispecies community of freshwater unionids. Canadian Journal of Zoology 78: 871–882.
Ostrovsky, A. N. & I. Y. Popov, 2011. Rediscovery of the largest population of the freshwater pearl mussel (Margaritifera margaritifera) in the Leningrad oblast (north-west Russia). Aquatic Conservation: Marine and Freshwater Ecosystems 21(2): 113–121.
Parmalee, P. W. & A. E. Bogan, 1998. The Freshwater Mussels of Tennessee. The University of Tennessee Press, Knoxville.
Raikow, D. F. & S. K. Hamilton, 2001. Bivalve diets in a midwestern U.S. stream: a stable isotope enrichment study. Limnnolgy and Oceanography 46: 514–522.
Spooner, D. E. & C. C. Vaughn, 2006. Context-dependent effects of freshwater mussels on stream benthic communities. Freshwater Biology 51: 1016–1024.
Spooner, D. E. & C. C. Vaughn, 2012. Species’ traits and environmental gradients interact to govern primary production in freshwater mussel communities. Oikos 121(3): 403–416.
Strayer, D. L. & D. R. Smith, 2003. A guide to sampling freshwater mussel populations. American Fisheries Society Monograph 8: 1–103.
Strickland, J. D. H. & T. R. Parsons, 1972. A Practical Handbook of Seawater Analysis.
Vanderploeg, H. A., J. R. Liebig & T. F. Nalepa, 1995. From picoplankton to microplankton: temperature-driven filtration by the unionid bivalve Lampsilis radiata siliquoidea in Lake St Clair. Canadian Journal of Fisheries and Aquatic Sciences 52: 63–74.
Vaughn, C. C., 2018. Ecosystem services provided by freshwater mussels. Hydrobiologia 810: 15–27.
Vaughn, C. C. & C. C. Hakenkamp, 2001. The functional role of burrowing bivalves in freshwater ecosystems. Freshwater Biology 46: 1431–1446.
Vaughn, C. C., K. B. Gido & D. E. Spooner, 2004. Ecosystem processes performed by unionid mussels in stream mesocosms: species roles and effects of abundance. Hydrobiologia 527: 35–47.
Walsh, E. J., H. A. Smith & R. L. Wallace, 2014. Rotifers of temporary waters. International Review of Hydrobiology 99(1–2): 3–19.
Weber, A. M., J. E. Bauer & G. T. Watters, 2017. Assessment of nutritional subsidies to freshwater mussels using a multiple natural abundance isotope approach. Freshwater Biology 62(3): 615–629.
Welker, M. & N. Walz, 1998. Can mussels control the plankton in rivers? A phytological approach to Lagrangian sampling strategy. American Society of Limnology and Oceanography 43: 753–762.
Yusoff, F. M., M. Shariff & N. Gopinath, 2006. Diversity of Malaysian aquatic ecosystems and resources. Aquatic Ecosystem Health & Management 9(2): 119–135.
Zieritz, A., M. Lopes-Lima, A. E. Bogan, R. Sousa, S. Walton, K. A. A. Rahim, J.-J. Wilson, P.-Y. Ng, E. Froufe & S. McGowan, 2016. Factors driving changes in freshwater mussel (Bivalvia, Unionida) diversity and distribution in Peninsular Malaysia. Science of the Total Environment 571: 1069–1078.
Zieritz, A., S. Azam-Ali, A. L. Marriott, N. A. B. M. Nasir, Q. N. Ng, N. A. A. B. A. Razak & M. Watts, 2018a. Biochemical composition of freshwater mussels in Malaysia: a neglected nutrient source for rural communities. Journal of Food Composition and Analysis 72: 104–114.
Zieritz, A., A. E. Bogan, E. Froufe, O. Klishko, T. Kondo, U. Kovitvadhi, S. Kovitvadhi, J. H. Lee, M. Lopes-Lima, J. M. Pfeiffer, R. Sousa, T. van Do, I. Vikhrev & D. T. Zanatta, 2018b. Diversity, biogeography and conservation of freshwater mussels (Bivalvia: Unionida) in East and Southeast Asia. Hydrobiologia 810: 29–44.
Zieritz, A., A. E. Bogan, K. A. A. Rahim, R. Sousa, L. Jainih, S. Harun, N. F. A. Razak, B. Gallardo, S. McGowan, R. Hassan & M. Lopes-Lima, 2018c. Changes and drivers of freshwater mussel diversity and distribution in northern Borneo. Biological Conservation 219: 126–137.
Zieritz, A., F. N. Mahadzir, W. N. Chan & S. McGowan, 2019. Effects of mussels on nutrient cycling and bioseston in two contrasting tropical freshwater habitats. Hydrobiologia 835: 179–191.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Manuel P. M. Lopes-Lima, Nicoletta Riccardi, Maria Urbanska & Ronaldo G. Sousa/Biology and Conservation of Freshwater Molluscs
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zieritz, A., Chan, W.N., McGowan, S. et al. High rates of biodeposition and N-excretion indicate strong functional effects of mussels (Bivalvia: Unionida) in certain anthropogenic tropical freshwater habitats. Hydrobiologia 848, 3153–3166 (2021). https://doi.org/10.1007/s10750-020-04464-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04464-y