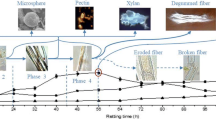
Abstract
The morphological features of raw ramie and the distribution of polysaccharides on such features were investigated using various microscopes to reveal the pivotal factors that influence microbial degumming efficiency. Results indicated that the morphological features mainly included fence, chunk, membrane, ribbon, and plank shapes. The various morphological features had their respective coverage characteristics. The fence and chunk shapes distributed on the membrane shape and cemented the fiber bundle. The ribbon shape was covered by the plank shape in several locations, and they covered one lateral surface of the fiber bundle as an integral. All of these morphological features were not observed in the refined fiber, indicating these features belonged to gum that consists of non-cellulosic components traditionally. However, some cellulose was also found in these features and should be classified as a new kind of gum component because this part of cellulose was not used for weaving. Apart from cellulose, the membrane shape contained mannan and xylan, and the fence and chunk shapes contained pectin, but the ribbon and plank shapes contained three of them. What’s more, the xylan on the membrane and ribbon shapes was masked by several components, and the mannan on the membrane and plank shapes was also masked. Furthermore, the coverage characteristics of the morphological features and the masking relationship (cross-linking) among polysaccharides influenced the contact degree between the enzyme and gum and further degumming efficiency. Increasing the contact degree between the enzyme and gum is a pivotal factor for improving degumming efficiency.
Graphic abstract










Similar content being viewed by others
Abbreviations
- OM:
-
Optic microscope
- SEM:
-
Scanning electron microscopy
- LSCM:
-
Laser scanning confocal microscope
- IF:
-
Immunofluorescence
- CBM3a:
-
Carbohydrate-binding module directed to crystalline cellulose
- LM10:
-
The primary antibodies specifically combined with xylan
- LM18:
-
The primary antibodies specifically combined with pectin
- LM21:
-
The primary antibodies specifically combined with mannan
References
Basu S, Saha MN, Chattopadhyay D et al (2009) Large-scale degumming of ramie fibre using a newly isolated Bacillus pumilus DKS1 with high pectate lyase activity. J Ind Microbiol Biotechnol 36:239–245
Blake AW, Mccartney L, Flint JE et al (2006) Understanding the biological rationale for the diversity of cellulose-directed carbohydrate-binding modules in prokaryotic enzymes. J Biol Chem 281(39):29321–29329
Blake AW, Marcus SE, Copeland JE et al (2008) In situ analysis of cell wall polymers associated with phloem fibre cells in stems of hemp, Cannabis sativa L.. Planta 228:1–13
Brühlmann F, Leupin M, Erismann KH et al (2000) Enzymatic degumming of ramie bast fibers. J Biotechnol 76:43–50
Bubner P, Plank H, Nidetzky B et al (2013) Visualizing cellulase activity. Biotechnol Bioeng 110:1529–1549
Chen Y, Sun LF, Chiparus O et al (2005) Kenaf/ramie composite for automotive headliner. J Polym Environ 13:107–114
Chernova TE, Mikshina PV, Salnikov VV et al (2018) Development of distinct cell wall layer both in primary and secondary phloem fibers of hemp (Cannabis sativa L.). Ind Crops Prod 117:97–109
Chu FK, Xu ZM, Mu XW et al (2020) Construction of hierarchical layered double hydroxide/poly (dimethylsiloxane) composite coatings on ramie fabric surfaces for oil/water separation and flame retardancy. Cellulose 27:3485–3499
Chundawat SPS, Donohoe BS, Sousa LD et al (2011) Multi-scale visualization and characterization of lignocellulosic plant cell wall deconstruction during thermochemical pretreatment. Energy Environ Sci 4:973–984
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:3–23
Cosgrove DJ (2014) Re-constructing our models of cellulose and primary cell wall assembly. Curr Opin Plant Biol 22:122–131
Cosgrove DJ, Jarvis MC (2012) Comparative structure and biomechanics of plant primary and secondary cell walls. Front Plant Sci 3:204
de Andrade EA, Folquitto DG, Luz LEC et al (2017) Anatomy and histochemistry of leaves and stems of Sapium glandulosum. Rev Bras Farmacogn 27:282–289
Ding SY, Liu YS, Zeng YN et al (2012) How does plant cell wall nanoscale architecture correlate with enzymatic digestibility. Science 338:1055–1060
Ding RY, Zhang XQ, Yu CW (2014) Optimization of enzyme mixture degumming of ramie fiber. J Nat Fibers 11:13–24
Duan SW, Liu ZC, Feng XY et al (2012) Diversity and characterization of ramie-degumming strains. Sci Agric 69:119–125
Fan P, He F, Yang Y et al (2015) In-situ microbial degumming technology with Bacillus sp. HG-28 for industrial production of ramie fibers. Biochem Eng J 97:50–58
Franceschi VR, Nakata PA (2005) Calcium oxalate in plants: formation and function. Annu Rev Plant Biol 56:41–71
Gilbert HJ (2010) The biochemistry and structural biology of plant cell wall deconstruction. Plant Physiol 153:444–455
Herve C, Rogowski A, Gilbert HJ et al (2009) Enzymatic treatments reveal differential capacities for xylan recognition and degradation in primary and secondary plant cell walls. Plant J 58:413–422
Himmel ME, Ding SY, Johnson DK et al (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 9:315
Hina KZ, Zou HT, Qian W et al (2018) Preparation and performance comparison of cellulose-based activated carbon fibres. Cellulose 1:607–617
Igarashi K, Koivula A, Wada M et al (2009) High speed atomic force microscopy visualizes processive movement of Trichoderma reesei Cellobiohydrolase I on crystalline cellulose. J Biol Chem 284:36186–36190
Igarashi K, Uchihashi T, Koivula A et al (2011) Traffic jams reduce hydrolytic efficiency of cellulase on cellulose surface. Science 2:333
Li ZF, Li ZL, Ding RY et al (2015) Composition of ramie hemicelluloses and effect of polysaccharides on fiber properties. Text Res J 86:451–460
Liu YS, Baker JO, Zeng YN et al (2011) Cellobiohydrolase hydrolyzes crystalline cellulose on hydrophobic faces. J Biol Chem 286:11195–11201
Manero JM, Gil FJ, Padrós E et al (2003) Applications of environmental scanning electron microscopy (ESEM) in biomaterials field. Microsc Res Tech 61:469–480
Mannan S, Paul KJ, Basu S (2017) Correlations between axial stiffness and microstructure of a species of bamboo. R Soc Open Sci 4:160412
Mao KW, Chen HG, Qi HH et al (2019) Visual degumming process of ramie fiber using a microbial consortium RAMCD407. Cellulose 26:3513–3528
Marcus SE, Verhertbruggen Y, Herve C et al (2008) Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol 8:60–60
Marcus SE, Blake AW, Benians TAS et al (2010) Restricted access of proteins to mannan polysaccharides in intact plant cell walls. Plant J 64:191–203
McCartney L, Marcus SE, Knox JP (2016) Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J Histochem Cytochem 53:543–546
Meng CR, Li ZL, Wang CY et al (2016) Sustained-release alkali source used in the oxidation degumming of ramie. Text Res J 87:1155–1164
Moreira LRS, Filho EXF (2008) An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol 79:165–178
Morvan C, Andème-Onzighi C, Girault R et al (2003) Building flax fibres: more than one brick in the walls. Plant Physiol Biochem 41:935–944
Pandey SN (2007a) Ramie fibre: part II. Physical fibre properties. A critical appreciation of recent developments. Text Prog 39:189–268
Pandey SN (2007b) Ramie fibre: part I. Chemical composition and chemical properties. A critical review of recent developments. Text Prog 39:1–66
Peng XY, Su SW, Xia MG et al (2018) Fabrication of carboxymethyl-functionalized porous ramie microspheres as effective adsorbents for the removal of cadmium ions. Cellulose 3:1921–1938
Pradipta B (2015) Evaluation of ramie fibre quality: a review. Int J Biores Sci 2:72–75
Qi HH, Chen HG, Mao KW et al (2019) Investigation of the structure of ramie fibers by enzymatic peeling. Cellulose 26:2955–2968
Saba N, Tahir PM, Jawaid M (2014) A Review on potentiality of nano filler/natural fiber filled polymer hybrid composites. Polymers 6:2247–2273
Schweingruber FH, Börner A (2018) The plant stem. Springer, Berlin. https://doi.org/10.1007/978-3-319-73524-5
Shu T, Bai Y, Wang YW et al (2020) A high-efficiency and eco-friendly degumming process for ramie fibers. J Clean Prod 276:124217
Tatyana A, Gorshkova SE, Wyatt VV et al (1996) Cell-wall polysaccharides of developing flax plants. Plant Physiol 110:721–729
Verhertbruggen Y, Marcus SE, Haeger A et al (2009) An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr Res 344:1858–1862
Wang YW, Shu T, Fan P et al (2017) Characterization of a recombinant alkaline thermostable β-mannanase and its application in eco-friendly ramie degumming. Process Biochem 61:73–79
Wang Q, Chen HG, Fang G et al (2017) Isolation of Bacillus cereus P05 and Pseudomonas sp. X12 and their application in the ramie retting. Ind Crops Prod 97:518–524
Wang YW, Bai Y, Shu T et al (2020) Characterization of a versatile glycosidehydrolase Cel5M from Pectobacterium carotovorum HG-49 for ramie degumming. Text Res J 90:1602–1615
Yang Q, Duan SW, Cheng LF et al (2019) Engineering of a Bacillus subtilis strain deficient in cellulase: application in degumming of ramie. Fibers Polym 20:57–62
Zheng LS, Du YM, Zhang JY (2000) Biobleaching effect of xylanase preparation from an alkalophilic Bacillus sp. on ramie fibers. Biotechnol Lett 22:1363–1367
Zheng LS, Du YM, Zhang JY (2001) Degumming of ramie fibers by alkalophilic bacteria and their polysaccharide-degrading enzymes. Biores Technol 78:89–94
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21676111). The analysis of OM, AFM and CLSM were carried out at the Research Core Facilities for Life Science (HUST). The SEM observation was finished at Analytical and Testing Center of Huazhong University of Science and Technology. The authors wish to express gratitude to their supports.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, P., Shu, T., Wang, H. et al. Visual analysis of the morphological features and polysaccharide distribution of raw ramie and their influence on degumming. Cellulose 28, 1203–1218 (2021). https://doi.org/10.1007/s10570-020-03599-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03599-4