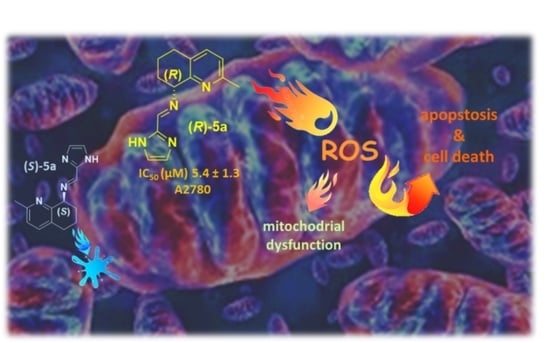
Biological Properties of New Chiral 2-Methyl-5,6,7,8-tetrahydroquinolin-8-amine-based Compounds
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. General Procedure for the Synthesis of Compounds 1a–6a
3.2. General Procedure for the Synthesis of Compounds 1b–6b
3.3. Cell Growth Assay
3.4. Cell Cycle Analysis
3.5. Evaluation of Mitochondrial Transmembrane Potential
3.6. Measurement of Reactive Oxygen Species (ROS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sekhon, B.S. Exploiting the power of stereochemistry in drugs: An overview of racemic and enantiopure drugs. J. Mod. Med. Chem. 2013, 1, 10–36. [Google Scholar] [CrossRef] [Green Version]
- Saha, D.; Kharbanda, A.; Yan, W.; Lakkaniga, N.R.; Frett, B.; Li, H.Y. The exploration of chirality for improved druggability within the human kinome. J. Med. Chem. 2019, 63, 441–469. [Google Scholar] [CrossRef] [PubMed]
- Uwai, Y. Enantioselective drug recognition by drug transporters. Molecules 2018, 23, 3062. [Google Scholar] [CrossRef] [Green Version]
- Calcaterra, A.; D’Acquarica, I. The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 2018, 147, 323–340. [Google Scholar] [CrossRef]
- De Camp, W.H. Chiral drugs: The FDA perspective on manufacturing and control. J. Pharm. Biomed. Anal. 1993, 11, 1167–1172. [Google Scholar] [CrossRef]
- Bentley, K.W. β-Phenylethylamines and the isoquinoline alkaloids. Nat. Prod. Rep. 2006, 23, 444–463. [Google Scholar] [CrossRef]
- Christodoulou, M.S.; Kasiotis, K.M.; Fokialakis, N.; Tellitu, I.; Haroutounian, S.A. PIFA-mediated synthesis of novel pyrazoloquinolin-4-ones as potential ligands for the estrogen receptor. Tetrahedron Lett. 2008, 49, 7100–7102. [Google Scholar] [CrossRef]
- Christodoulou, M.S.; Liekens, S.; Kasiotis, K.M.; Haroutounian, S.A. Novel pyrazole derivatives: Synthesis and evaluation of anti-angiogenic activity. Bioorganic Med. Chem. 2010, 18, 4338–4350. [Google Scholar] [CrossRef]
- Beccalli, E.M.; Broggini, G.; Martinelli, M.; Masciocchi, N.; Sottocornola, S. New 4-spiroannulated tetrahydroisoquinolines by a one-pot sequential procedure. Isolation and characterization of σ-alkylpalladium Heck intermediates. Org. Lett. 2006, 8, 4521–4524. [Google Scholar] [CrossRef]
- Mantu, D.; Antoci, V.; Moldoveanu, C.; Zbancioc, G.; Mangalagiu, I. Hybrid imidazole (benzimidazole)/pyridine (quinoline) derivatives and evaluation of their anticancer and antimycobacterial activity. J. Enzym. Inhib. Med. Chem. 2016, 31, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Chiarelli, L.R.; Mori, M.; Barlocco, D.; Beretta, G.; Gelain, A.; Pini, E.; Porcino, M.; Mori, G.; Stelitano, G.; Costantino, L.; et al. Discovery and development of novel salicylate synthase (MbtI) furanic inhibitors as antitubercular agents. Eur. J. Med. Chem. 2018, 155, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Basilico, N.; Migotto, M.; Ilboudo, D.P.; Taramelli, D.; Stradi, R.; Pini, E. Modified quaternary ammonium salts as potential antimalarial agents. Bioorganic Med. Chem. 2015, 23, 4681–4687. [Google Scholar] [CrossRef] [PubMed]
- Villa, S.; Legnani, L.; Colombo, D.; Gelain, A.; Lammi, C.; Bongiorno, D.; Ilboudo, D.P.; McGee, K.E.; Bosch, J.; Grazioso, G. Structure-based drug design, synthesis and biological assays of P. falciparum Atg3–Atg8 protein–protein interaction inhibitors. J. Comput. Aided Mol. Des. 2018, 32, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, L.R.; Mori, M.; Beretta, G.; Gelain, A.; Pini, E.; Sammartino, J.C.; Stelitano, G.; Barlocco, D.; Costantino, L.; Lapillo, M.; et al. New insight into structure-activity of furan-based salicylate synthase (MbtI) inhibitors as potential antitubercular agents. J. Enzym. Inhib. Med. Chem. 2019, 34, 823–828. [Google Scholar] [CrossRef] [Green Version]
- Facchetti, G.; Ferri, N.; Lupo, M.G.; Giorgio, L.; Rimoldi, I.; Lucchini, G. Monofunctional PtII Complexes based on 8-aminoquinoline: Synthesis and pharmacological characterization. Eur. J. Inorg. Chem. 2019, 2019, 3389–3395. [Google Scholar] [CrossRef]
- Facchetti, G.; Rimoldi, I. 8-Amino-5,6,7,8-tetrahydroquinoline in iridium(iii) biotinylated Cp* complex as artificial imine reductase. New J. Chem. 2018, 42, 18773–18776. [Google Scholar] [CrossRef]
- Facchetti, G.; Bucci, R.; Fusè, M.; Rimoldi, I. Asymmetric hydrogenation vs transfer hydrogenation in the reduction of cyclic imines. ChemistrySelect 2018, 3, 8797–8800. [Google Scholar] [CrossRef]
- Ricciardi, L.; La Deda, M.; Ionescu, A.; Godbert, N.; Aiello, I.; Ghedini, M.; Fusè, M.; Rimoldi, I.; Cesarotti, E. Luminescent chiral ionic Ir(III) complexes: Synthesis and photophysical properties. J. Lumin. 2016, 170, 812–819. [Google Scholar] [CrossRef]
- Zerla, D.S.; Rimoldi, I.; Cesarotti, E.; Facchetti, G.; Pellizzoni, M.M.; Fusè, M. Diastereoselectivity and catalytic activity in ruthenium complexes chiral at the metal centre. J. Organomet. Chem. 2014, 771, 2–8. [Google Scholar] [CrossRef]
- Zerla, D.; Facchetti, G.; Fusè, M.; Pellizzoni, M.M.; Castellano, C.; Cesarotti, E.; Gandolfi, R.; Rimoldi, I. 8-Amino-5,6,7,8-tetrahydroquinolines as ligands in iridium(III) catalysts for the reduction of aryl ketones by asymmetric transfer hydrogenation (ATH). Tetrahedron Asymmetry 2014, 25, 1031–1037. [Google Scholar] [CrossRef]
- Rimoldi, I.; Facchetti, G.; Cesarotti, E.; Pellizzoni, M.M.; Fusè, M.; Zerla, D. Enantioselective transfer hydrogenation of aryl ketones: Synthesis and 2D-NMR characterization of new 8-amino-5,6,7,8-tetrahydroquinoline Ru(II)-complexes. Curr. Org. Chem. 2012, 16, 2982–2988. [Google Scholar] [CrossRef]
- Abbiati, G.; Beccalli, E.M.; Broggini, G.; Zoni, C. A valuable heterocyclic ring transformation: From isoxazolin-5(2H)-ones to quinolines. Tetrahedron 2003, 59, 9887–9893. [Google Scholar] [CrossRef]
- Saitoh, T.; Abe, K.; Ishikawa, M.; Nakatani, M.; Shimazu, S.; Satoh, N.; Yoneda, F.; Taguchi, K.; Horiguchi, Y. Synthesis and in vitro cytotoxicity of 1,2,3,4-tetrahydroisoquinoline derivatives. Eur. J. Med. Chem. 2006, 41, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.Y.L.; Lam, P.L.; Zhou, Y.; Gasparello, J.; Finotti, A.; Chilin, A.; Marzaro, G.; Gambari, R.; Bian, Z.; Kwok, W.M.; et al. Targeting DNA binding for NF-κB as an anticancer approach in Hepatocellular Carcinoma. Cells 2018, 7, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facchetti, G.; Rimoldi, I. Anticancer platinum(II) complexes bearing N-heterocycle rings. Bioorganic Med. Chem. Lett. 2019, 29, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Vivar, J.; Augusto, O. Oxidative activity of primaquine metabolites on rat erythrocytes in vitro and in vivo. Biochem. Pharmacol. 1994, 47, 309–316. [Google Scholar] [CrossRef]
- Zorc, B.; Perković, I.; Pavić, K.; Rajić, Z.; Beus, M. Primaquine derivatives: Modifications of the terminal amino group. Eur. J. Med. Chem. 2019, 182, 111640. [Google Scholar] [CrossRef]
- Pavić, K.; Perković, I.; Pospíšilová, Š.; Machado, M.; Fontinha, D.; Prudêncio, M.; Jampilek, J.; Coffey, A.; Endersen, L.; Rimac, H.; et al. Primaquine hybrids as promising antimycobacterial and antimalarial agents. Eur. J. Med. Chem. 2018, 143, 769–779. [Google Scholar]
- Hu, S.; Luesakul, U.; Liu, Y.; Ljungman, M.; Neamati, N. Synthesis and mechanistic studies of quinolin-chlorobenzothioate derivatives with proteasome inhibitory activity in pancreatic cancer cell lines. Eur. J. Med. Chem. 2018, 158, 884–895. [Google Scholar] [CrossRef]
- Sutherland, J.J.; Raymond, J.W.; Stevens, J.L.; Baker, T.K.; Watson, D.E. Relating molecular properties and in vitro assay results to in vivo drug disposition and toxicity outcomes. J. Med. Chem. 2012, 55, 6455–6466. [Google Scholar] [CrossRef]
- Celli, C.M.; Tran, N.; Knox, R.; Jaiswal, A.K. NRH: Quinone oxidoreductase 2 (NQO2) catalyzes metabolic activation of quinones and anti-tumor drugs. Biochem. Pharmacol. 2006, 72, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.J.; Jecs, E.; Truax, V.M.; Katzman, B.M.; Tahirovic, Y.A.; Wilson, R.J.; Kuo, K.M.; Kim, M.B.; Nguyen, H.H.; Saindane, M.T.; et al. Discovery of tetrahydroisoquinoline-containing CXCR4 antagonists with improved in vitro ADMET properties. J. Med. Chem. 2018, 61, 946–979. [Google Scholar] [CrossRef] [PubMed]
- Katzman, B.M.; Cox, B.D.; Prosser, A.R.; Alcaraz, A.A.; Murat, B.; Heroux, M.; Tebben, A.J.; Zhang, Y.; Schroeder, G.M.; Snyder, J.P.; et al. Tetrahydroisoquinoline CXCR4 antagonists adopt a hybrid binding mode within the peptide subpocket of the CXCR4 receptor. ACS Med. Chem. Lett. 2018, 10, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Jantová, S.; Repický, A.; Letašiová, S.; Čipák, L. 4-Amino-3-acetylquinoline-induced apoptosis of murine L1210 leukemia cells involves ROS-mitochondrial-mediated death signaling and activation of p38 MAPK. Cell Biochem. Funct. 2008, 26, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, H.; Chen, W.; Zhan, P.; Liu, X. 8-Hydroxyquinoline: A privileged structure with a broad-ranging pharmacological potential. MedChemComm 2015, 6, 61–74. [Google Scholar] [CrossRef]
- Ling, Y.; Yang, Q.-X.; Teng, Y.-N.; Chen, S.; Gao, W.-J.; Guo, J.; Hsu, P.-L.; Liu, Y.; Morris-Natschke, S.L.; Hung, C.-C.; et al. Development of novel amino-quinoline-5,8-dione derivatives as NAD(P)H:quinone oxidoreductase 1 (NQO1) inhibitors with potent antiproliferative activities. Eur. J. Med. Chem. 2018, 154, 199–209. [Google Scholar] [CrossRef]
- Gandolfi, R.; Facchetti, G.; Christodoulou, M.S.; Fusè, M.; Meneghetti, F.; Rimoldi, I. Cascade reaction by chemo- and biocatalytic approaches to obtain chiral hydroxy ketones and anti 1,3-diols. ChemistryOpen 2018, 7, 393–400. [Google Scholar] [CrossRef]
- Organisation for Economic Cooperation and Development. In OECD Guideline for Testing of Chemicals-Partition Coefficient (n-Octanol/Water), High Performance Liquid Chromatography (HPLC) Method, 117, Adopted: 30.03.89; Organisation for Economic Cooperation and Development: Paris, France, 2004.
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Xing, L.; Glen, R.C. Novel methods for the prediction of logP, pK(a), and logD. J. Chem. Inf. Comput. Sci. 2008, 42, 796–805. [Google Scholar] [CrossRef]
- Christodoulou, M.S.; Calogero, F.; Baumann, M.; Garcia-Argaez, A.N.; Pieraccini, S.; Sironi, M.; Dapiaggi, F.; Bucci, R.; Broggini, G.; Gazzola, S.; et al. Boehmeriasin A as new lead compound for the inhibition of topoisomerases and SIRT2. Eur. J. Med. Chem. 2015, 92, 766–775. [Google Scholar] [CrossRef]
- Zidar, N.; Secci, D.; Tomašič, T.; Mašič, L.P.; Kikelj, D.; Passarella, D.; Argaez, A.N.G.; Hyeraci, M.; Dalla Via, L. Synthesis, antiproliferative effect, and topoisomerase ii inhibitory activity of 3-methyl-2-phenyl-1H-indoles. ACS Med. Chem. Lett. 2020, 11, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Uenishi, J.; Hamada, M. Synthesis of enantiomerically pure 8-substituted 5,6,7,8-tetrahydroquinolines. Synthesis 2002, 2002, 0625–0630. [Google Scholar] [CrossRef]
- Delgado, J.L.; Hsieh, C.M.; Chan, N.-L.; Hiasa, H. Topoisomerases as anticancer targets. Biochem. J. 2018, 475, 373–398. [Google Scholar] [CrossRef] [PubMed]
- Mitra, K.; Wunder, C.; Roysam, B.; Lin, G.; Lippincott-Schwartz, J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc. Natl. Acad. Sci. USA 2009, 106, 11960–11965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cossarizza, A.; Baccaranicontri, M.; Kalashnikova, G.; Franceschi, C. A new method for the cytofluorometric analysis of mitochondrial membrane potential using the j-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1). Biochem. Biophys. Res. Commun. 1993, 197, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Bucci, R.; Bonetti, A.; Clerici, F.; Contini, A.; Nava, D.; Pellegrino, S.; Tessaro, D.; Gelmi, M.L. Tandem tetrahydroisoquinoline-4-carboxylic acid/β-Alanine as a new construct able to induce a flexible turn. Chem. Eur. J. 2017, 23, 10822–10831. [Google Scholar] [CrossRef]





| Compound | IC50 (µM) | ||
|---|---|---|---|
| CEM | HeLa | HMEC-1 | |
| 1a | >100 | >100 | >100 |
| 2a | >100 | >100 | >100 |
| 3a | 33 ± 7 | 34 ± 6 | 77 ± 32 |
| 4a | >100 | >100 | >100 |
| 5a | 43 ± 7 | 37 ± 9 | 57 ± 4 |
| 6a | 64 ± 32 | >100 | 82 ± 4 |
| 1b | 66 ± 47 | 93 ± 10 | 63 ± 2 |
| 2b | 23 ± 4 | 75 ± 11 | 60 ± 2 |
| 3b | >100 | >100 | >100 |
| 4b | >100 | >100 | 89 ± 2 |
| 5b | 51 ± 13 | 71 ± 3 | 53 ± 0 |
| 6b | 77 ± 32 | >100 | 81 ± 26 |
| CA-4P a | 0.095 ± 0.006 | 0.079 ± 0.003 | 0.0029 ± 0 |
| Compound | IC50 (µM) | ||
|---|---|---|---|
| HT-29 | A2780 | MSTO-211H | |
| 1a | 83 ± 5 | 65 ± 9 | 69 ± 3 |
| 2a | >100 | >100 | 95 ± 5 |
| 3a | 42 ± 6 | 16 ± 2 | 30 ± 8 |
| 4a | 42 ± 8 | 11 ± 2 | 14 ± 1 |
| 5a | 70 ± 2 | 13 ± 3 | 31 ± 7 |
| 6a | 46 ± 7 | 36 ± 4 | 25 ± 11 |
| 1b | 57 ± 6 | 42 ± 5 | 28 ± 4 |
| 2b | 62 ± 8 | 25 ± 5 | 35 ± 10 |
| 3b | 42 ± 6 | 11 ± 3 | 15 ± 3 |
| 4b | >100 | >100 | >100 |
| 5b | 73 ± 6 | 38 ± 7 | 44 ± 5 |
| 6b | 74 ± 3 | 36 ± 4 | 77 ± 7 |
| m-AMSA a | 0.039 ± 0.004 | 0.027 ± 0.005 a | 0.019 ± 0.001 a |
| Compound | IC50 (µM) | ||
|---|---|---|---|
| HT-29 | A2780 | MSTO-211H | |
| (R)-3a | >20 | 11.7 ± 2 | 14.9 ± 1.4 |
| (S)-3a | >20 | 11.4 ± 0.4 | 11.8 ± 2.3 |
| (R)-5a | >20 | 5.4 ± 1.3 | 15.1 ± 1.5 |
| (S)-5a | >20 | 17.2 ± 3 | >20 |
| (R)-2b | >20 | 15.2 ± 2.3 | >20 |
| (S)-2b | >20 | 11.5 ± 2.6 | >20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facchetti, G.; Christodoulou, M.S.; Mendoza, L.B.; Cusinato, F.; Dalla Via, L.; Rimoldi, I. Biological Properties of New Chiral 2-Methyl-5,6,7,8-tetrahydroquinolin-8-amine-based Compounds. Molecules 2020, 25, 5561. https://doi.org/10.3390/molecules25235561
Facchetti G, Christodoulou MS, Mendoza LB, Cusinato F, Dalla Via L, Rimoldi I. Biological Properties of New Chiral 2-Methyl-5,6,7,8-tetrahydroquinolin-8-amine-based Compounds. Molecules. 2020; 25(23):5561. https://doi.org/10.3390/molecules25235561
Chicago/Turabian StyleFacchetti, Giorgio, Michael S. Christodoulou, Lina Barragán Mendoza, Federico Cusinato, Lisa Dalla Via, and Isabella Rimoldi. 2020. "Biological Properties of New Chiral 2-Methyl-5,6,7,8-tetrahydroquinolin-8-amine-based Compounds" Molecules 25, no. 23: 5561. https://doi.org/10.3390/molecules25235561