Abstract
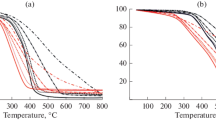
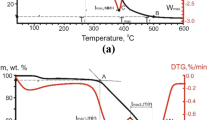
Adding copper nitrate Cu(NO3)2 is known to activate the oxidation of coal and lignite. In the present work, the change in its activating properties with increase in the heating rate is studied. The Cu(NO3)2 is first dissolved in a 50/50 (by volume) mixture of ethyl alcohol and water. Then it is applied to the fuel by steeping, to a content of 5 wt %. Activated oxidation is studied by thermal analysis, with different heating rates (2.5, 10, 20, and 40°C/min) in the temperature range 25–1000°C, at atmospheric pressure. With increase in heating rate, the catalytic effect of the additive is intensified: the initial (30–115°C) and final (85–180°C) oxidation temperatures fall, with increase in the maximum reaction rate. Mass-spectrometric analysis of the gaseous oxidation products shows that, in the presence of copper nitrate, increase in the heating rate leads to greater CO2 emission, with shorter oxidation time. The dependence of the activation energy on the degree of fuel conversion is determined by the Friedman method. The decrease in the mean activation energy when using Cu(NO3)2 is ~14 kJ/mol for lignite and ~35 kJ/mol for coal.




Similar content being viewed by others
REFERENCES
Korolev, A.V. and Lomakina, N.S., The role of coal in the fuel and energy complex, Sovrem. Naukoemkie Tekhnol., 2013, no. 8, pp. 125–126.
Parmon, V.N., Simonov, A.D., Sadykov, V.A., and Tikhov, S.F., Catalytic combustion: Achievements and problems, Combust., Explos. Shock Waves (Engl. Transl.), 2015, vol. 51, no. 2, pp. 143–150.
Sidorov, A.D., Fedorov, I.A., Dubinin, Yu.V., et al., Catalytic thermal systems for industrial heating, Katal. Prom-sti, 2012, no. 3, pp. 50–57.
Gong, X., Guo, Z., and Wang, Z., Reactivity of pulverized coals during combustion catalyzed by CeO2 and Fe2O3, Combust. Flame, 2010, vol. 157, pp. 351–356.
Gong, X., Guo, Z., and Wang, Z., Variation on anthracite combustion efficiency with CeO2 and Fe2O3 addition by differential thermal analysis (DTA), Energy, 2010, vol. 35, pp. 506–511.
Gong, X., Guo, X., and Wang, Z., Variation of char structure during anthracite pyrolysis catalyzed by Fe2O3 and its influence on char combustion reactivity, Energy Fuels, 2009, vol. 23, pp. 4547–4552.
Larionov, K.B. and Gromov, A.A., Non-isothermal oxidation of coal with Ce(NO3)3 and Cu(NO3)2 additives, Int. J. Coal Sci. Technol., 2019, vol. 6, no. 1, pp. 37–50.
Larionov, K.B., Mishakov, I.V., Vedyagin, A.A., and Gubin, V.E., Effect of an initiating additive of CuSO4 on changes in the characteristics of brown coal oxidation and pyrolysis, Solid Fuel Chem., 2019, vol. 53, no. 2, pp. 120–127.
Larionov, K.B., Mishakov, I.V., Slyusarskii, K.V., et al., Intensification of the oxidation of lignite and coal by an activating additive of Fe(NO3)2, Solid Fuel Chem., 2019, vol. 53, no. 5, pp. 262–269.
Tokareva, I.V., Mishakov, I.V., Korneev, D.V., et al., Nanostructuring of the carbon macrofiber surface, Nanotechnol. Russ., 2015, vol. 10, pp. 158–164.
Kok, M.V., An investigation into the thermal behavior of coals, Energy Source, 2002, vol. 24, pp. 899–906.
Santos, J.C.O., Oliveria, A.D., Silva, C.C., et al., Kinetic and activation thermodynamic parameters on thermal decomposition of synthetic lubricant oils, J. Therm. Anal. Calorim., 2007, vol. 87, pp. 823–829.
Vyazovkin, S., Burnham, A.K., Criado, J.M., et al., ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data, Thermochim. Acta, 2011, vol. 520, pp. 1–19.
Yang, F., Lai, Y., and Song, Y., Determination of the influence of pyrite on coal spontaneous combustion by thermodynamics analysis, Process Saf. Environ. Prot., 2019, vol. 129, pp. 163–167.
Wang, Y., Wang, J., Chen, H., et al., Preparation and NOx-assisted soot oxidation activity of a CuO–CeO2 mixed oxide catalyst, Chem. Eng. Sci., 2015, vol. 135, pp. 294–300.
Larionov, K.B., Tsibulskiy, S.A., Slyusarskiz, K.V., et al., Influence of inorganic salt on the characteristics of oxidation, ignition and combustion of bituminous coal, J. Phys.: Conf. Ser., 2019, vol. 1359, no. 1, art. ID 012065.
Funding
The research was carried out within the framework of the State Assignment no. 075-00268-20-02 (ID: 0718-2020-0040) and Russian President Scientific School NSh 2513.2020.8 executed at the National University of Science and Technology MISIS.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by B. Gilbert
About this article
Cite this article
Larionov, K.B., Mishakov, I.V., Zenkov, A.V. et al. Influence of the Heating Rate on the Activation of Coal and Lignite Oxidation by Copper Nitrate. Coke Chem. 63, 357–362 (2020). https://doi.org/10.3103/S1068364X20080037
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068364X20080037