Abstract
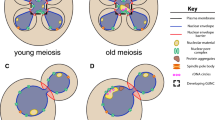
Cell aging is the result of deteriorating competence in maintaining cellular homeostasis and quality control. Certain cell types are able to rejuvenate through asymmetric cell division by excluding aging factors, including damaged cellular compartments and extrachromosomal rDNA circles, from entering the daughter cell. Recent findings from the budding yeast S. cerevisiae have shown that gametogenesis represents another type of cellular rejuvenation. Gametes, whether produced by an old or a young mother cell, are granted a renewed replicative lifespan through the formation of a fifth nuclear compartment that sequesters the harmful senescence factors accumulated by the mother. Here, we describe the importance and mechanism of cellular remodeling at the nuclear envelope mediated by ESCRT-III and the LEM-domain proteins, with a focus on nuclear pore biogenesis and chromatin interaction during gamete rejuvenation.

Similar content being viewed by others
References
Arai K, Sato M, Tanaka K, Yamamoto M (2010) Nuclear compartmentalization is abolished during fission yeast meiosis. Curr Biol 20:1913–1918
Asakawa H, Kojidani T, Mori C, Osakada H, Sato M, Ding DQ, Hiraoka Y, Haraguchi T (2010) Virtual breakdown of the nuclear envelope in fission yeast meiosis. Curr Biol 20:1919–1925
Beck M, Hurt E (2017) The nuclear pore complex: understanding its function through structural insight. Nat Rev Mol Cell Biol 18:73–89
Chu S, DeRisi J, Eisen M, Mulholland J, Botstein D, Brown PO, Herskowitz I (1998) The transcriptional program of sporulation in budding yeast. Science 282:699–705
Colombi P, Webster BM, Frohlich F, Lusk CP (2013) The transmission of nuclear pore complexes to daughter cells requires a cytoplasmic pool of Nsp1. J Cell Biol 203:215–232
Dey G, Culley S, Curran S, Schmidt U, Henriques R, Kukulski W, Baum B (2020) Closed mitosis requires local disassembly of the nuclear envelope. Nature 585:119–123
Dultz E, Zanin E, Wurzenberger C, Braun M, Rabut G, Sironi L, Ellenberg J (2008) Systematic kinetic analysis of mitotic dis- and reassembly of the nuclear pore in living cells. J Cell Biol 180:857–865
Fernandez-Alvarez A, Bez C, O’Toole ET, Morphew M, Cooper JP (2016) Mitotic nuclear envelope breakdown and spindle nucleation are controlled by interphase contacts between centromeres and the nuclear envelope. Dev Cell 39:544–559
Fuchs J, Loidl J (2004) Behaviour of nucleolus organizing regions (NORs) and nucleoli during mitotic and meiotic divisions in budding yeast. Chromosome Res 12:427–438
Grund SE, Fischer T, Cabal GG, Antunez O, Perez-Ortin JE, Hurt E (2008) The inner nuclear membrane protein Src1 associates with subtelomeric genes and alters their regulated gene expression. J Cell Biol 182:897–910
Kaeberlein M (2010) Lessons on longevity from budding yeast. Nature 464:513–519
Kim SJ, Fernandez-Martinez J, Nudelman I, Shi Y, Zhang W, Raveh B, Herricks T, Slaughter BD, Hogan JA, Upla P, Chemmama IE, Pellarin R, Echeverria I, Shivaraju M, Chaudhury AS, Wang J, Williams R, Unruh JR, Greenberg CH, Jacobs EY, Yu Z, de la Cruz MJ, Mironska R, Stokes DL, Aitchison JD, Jarrold MF, Gerton JL, Ludtke SJ, Akey CW, Chait BT, Sali A, Rout MP (2018) Integrative structure and functional anatomy of a nuclear pore complex. Nature 555:475–482
King GA, Unal E (2020) The dynamic nuclear periphery as a facilitator of gamete health and rejuvenation. Curr Genet 8:1–7
King GA, Goodman JS, Schick JG, Chetlapalli K, Jorgens DM, McDonald KL, Unal E (2019) Meiotic cellular rejuvenation is coupled to nuclear remodeling in budding yeast. Elife 8:e47156
Knockenhauer KE, Schwartz TU (2016) The nuclear pore complex as a flexible and dynamic gate. Cell 164:1162–1171
Koch BA, Staley E, Jin H, Yu HG (2020) The ESCRT-III complex is required for nuclear pore complex sequestration and regulates gamete replicative lifespan in budding yeast meiosis. Nucleus 11:219–236
Longo VD, Shadel GS, Kaeberlein M, Kennedy B (2012) Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab 16:18–31
Makio T, Lapetina DL, Wozniak RW (2013) Inheritance of yeast nuclear pore complexes requires the Nsp1p subcomplex. J Cell Biol 203:187–196
Markossian S, Suresh S, Osmani AH, Osmani SA (2015) Nup2 requires a highly divergent partner, NupA, to fulfill functions at nuclear pore complexes and the mitotic chromatin region. Mol Biol Cell 26:605–621
Mekhail K, Seebacher J, Gygi SP, Moazed D (2008) Role for perinuclear chromosome tethering in maintenance of genome stability. Nature 456:667–670
Meszaros N, Cibulka J, Mendiburo MJ, Romanauska A, Schneider M, Kohler A (2015) Nuclear pore basket proteins are tethered to the nuclear envelope and can regulate membrane curvature. Dev Cell 33:285–298
Neiman AM (2011) Sporulation in the budding yeast Saccharomyces cerevisiae. Genetics 189:737–765
Pieper GH, Sprenger S, Teis D, Oliferenko S (2020) ESCRT-III/Vps4 controls heterochromatin-nuclear envelope attachments. Dev Cell 53(27–41):e26
Sinclair D, Mills K, Guarente L (1998) Aging in Saccharomyces cerevisiae. Annu Rev Microbiol 52:533–560
Suresh S, Markossian S, Osmani AH, Osmani SA (2017) Mitotic nuclear pore complex segregation involves Nup2 in Aspergillus nidulans. J Cell Biol 216:2813–2826
Thaller DJ, Allegretti M, Borah S, Ronchi P, Beck M, Lusk CP (2019) An ESCRT-LEM protein surveillance system is poised to directly monitor the nuclear envelope and nuclear transport system. Elife. 8:e45284
Unal E, Amon A (2011) Gamete formation resets the aging clock in yeast. Cold Spring Harb Symp Quant Biol 76:73–80
Unal E, Kinde B, Amon A (2011) Gametogenesis eliminates age-induced cellular damage and resets life span in yeast. Science 332:1554–1557
Ungricht R, Kutay U (2015) Establishment of NE asymmetry-targeting of membrane proteins to the inner nuclear membrane. Curr Opin Cell Biol 34:135–141
von Appen A, LaJoie D, Johnson IE, Trnka MJ, Pick SM, Burlingame AL, Ullman KS, Frost A (2020) LEM2 phase separation promotes ESCRT-mediated nuclear envelope reformation. Nature 582:115–118
Webster BM, Colombi P, Jager J, Lusk CP (2014) Surveillance of nuclear pore complex assembly by ESCRT-III/Vps4. Cell 159:388–401
Webster BM, Thaller DJ, Jager J, Ochmann SE, Borah S, Lusk CP (2016) Chm7 and Heh1 collaborate to link nuclear pore complex quality control with nuclear envelope sealing. EMBO J 35:2447–2467
Acknowledgements
We thank Charles Badland and Jen Kennedy for their assistance. The work in authors’ lab is supported by the National Institutes of Health GM138838 and the National Science Foundation MCB1951313.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M.Kupiec.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koch-Bojalad, B.A., Carson, L. & Yu, HG. Forever young: the key to rejuvenation during gametogenesis. Curr Genet 67, 231–235 (2021). https://doi.org/10.1007/s00294-020-01133-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-020-01133-4