Abstract
Eusocial bees are likely to be ecologically important competitors for floral resources, although competitive effects can be difficult to quantify in wild pollinator communities. To investigate this, we excluded honeybees (HBE treatment), bumblebees (BBE) or both (HB&BBE) from wild-growing patches of bramble, Rubus fruticosus L. agg., flowers in two eight-day field trials at separate locations, with complementary mapping of per-site local floral resource availability. Exclusions increased per-flower volume of nectar and visitation rates of non-excluded bees, compared to control patches with no bee exclusions (CON). There was a large increase in average nectar standing crop volume both at Site 1 (+ 172%) and Site 2 (+ 137%) in HB&BBE patch flowers, and no significant change in HBE or BBE, compared to CON patches. Foraging bee responses to exclusion treatments were more pronounced at Site 2, which may be due to lower local floral resource availability, since this is likely to increase the degree of exploitative competition present. Notably, at Site 2, there was a 447% increase in larger-bodied solitary (non-Apis/Bombus) bees visiting HB&BBE patches, suggesting ecological release from competition. Hoverflies showed no response to bee removals. Numbers of other non-bee insect groups were very small and also showed no clear response to exclusions. Our findings reveal patterns of competitive exclusion between pollinator groups, mediated by resource depletion by eusocial bees. Possible long-term implications of displacement from preferred flowers, particularly where alternative forage is reduced, are discussed.
Significance statement
Understanding patterns of exploitative competition and displacement is necessary for pollinator conservation, particularly for vulnerable or threatened species. In this research, experimental methods reveal underlying patterns of resource competition exerted by eusocial bees in a wild pollinator community. We show that honeybees and bumblebees competitively displace each other and particularly solitary (non-Apis/Bombus) bees from bramble, an important native nectar and pollen source. Effects were stronger where local floral resource availability was identified to be limited. Notably, following experimental exclusion of both honey- and bumblebees from flowers, visitation by solitary bees increased by up to 447%, strongly suggesting ecological release from competition. These results highlight the need for informed landscape management for pollinator wellbeing, including appropriate honeybee stocking densities and improved floral resource availability.
Similar content being viewed by others
Introduction
Flower-visiting insects gather pollen and nectar for their energetic needs and to feed their larvae. Because these resources are shared and can also be limiting, this sets the stage for exploitative competition both among individuals and species of flower-visitors. Depletion of floral resources by dominant foragers can cause others to move to alternative sources of forage, in both spatial and temporal partitioning of resources (Wilms and Wiechers 1997; Walther-Hellwig et al. 2006; Dupont et al. 2004). This can mitigate the negative effects of competition, allowing coexistence (Amarasekare 2003). However, and particularly where resources are limited, there may be fitness costs for non-dominant species following reduced overall resource acquisition and a decrease in reproductive output (Zurbuchen et al. 2010a; Hudewenz and Klein 2015; Thomson 2016).
Honeybees (Apis mellifera L.) and bumblebees (Bombus spp.) are relatively abundant in many flower-visiting insect communities (e.g. Nielsen et al. 2012; Garbuzov and Ratnieks 2014; Balfour et al. 2015a). Both have large colony requirements for nectar and pollen (Seeley 1995; Cane and Tepedino 2016; Rotheray et al. 2017), which foragers extract from flowers more rapidly than many other insects, including solitary bee species (Couvillon et al. 2015). They have large foraging ranges allowing them to maximise resource use over a wide area (Seeley 1995; Walther-Hellwig and Frankl 2000), and honeybees are able to actively recruit nestmates to profitable resources via the waggle dance (Seeley 1995). Additionally, Bombus and particularly Apis are often commercially managed for pollination or (Apis) honey production, which can create artificially high densities particularly of honeybees. Overall, they are likely to deplete floral resources where they are present (Torné-Noguera et al. 2016), thereby exerting competitive pressure on other flower-visiting insects. This has received continuing research interest (for example, see recent reviews: Mallinger et al. 2017 and Wojcik et al. 2018).
Resource depletion by honey- and bumblebees can have negative effects between these groups (Goulson and Sparrow 2009; Thomson 2016; Sáez et al. 2017). It can also affect the many species of non-Apis/Bombus bees (e.g. Hudewenz and Klein 2015), most of which have a solitary lifecycle and are hereafter termed ‘solitary bees’ (following Baldock et al. 2015, Balfour et al. 2015a, Baldock et al. 2019). Female solitary bees build a central nest to rear their offspring, provisioning each cell with pollen and nectar. They tend to have much smaller foraging distances than honey- and bumblebee workers (Gathmann and Tscharntke 2002; Zurbuchen et al. 2010b), meaning that they may be more affected by local changes in resource availability. In one study, experimentally increasing the distance from two species of solitary bee (Hoplitis adunca Panzer and Chelostoma rapunculi Lepeletier) nests to preferred flowers increased the duration of foraging bouts for the same quantity of resources. The authors used an indirect method to show that increased foraging duration caused a decrease in reproductive output (Zurbuchen et al. 2010a). Solitary bees may therefore be particularly vulnerable to the effects of exploitative competition, particularly when resources are scarce. However, this is not well understood (see Mallinger et al. 2017), despite the importance of these bees for the pollination of many crop and wild plants (Williams and Kremen 2007; Garratt et al. 2014; Garibaldi et al. 2014; Mallinger and Gratton 2015).
Apis/Bombus-mediated depletion of nectar and pollen may also affect other flower-visiting insects such as hoverflies, non-syrphid dipteran flies, butterflies, moths and wasps. Excepting wasps, these groups are not central-place foragers, i.e. they do not have a central nest to provision. Their foraging strategies and requirements are therefore different to bees, since they do not collect resources to feed offspring, having only individual energetic needs, and can also more flexibly move away from areas where resources are depleted. As such, it is possible that their foraging behaviour may be less affected by immediate local resource depletion. Previous research found that an increase in honeybee abundance caused spatial displacement of bumblebees, solitary bees and non-bee flying insects in oilseed crop fields (Lindström et al. 2016). However, there is little research investigating the effects of exploitative competition on non-bee insects.
The effects of resource competition are often studied where managed pollinators are introduced to areas where they are not native or are uncommon, thereby creating an increase in the abundance of a potential competitor (reviewed in Stout and Morales 2009). However, patterns of competition in natural flower-visiting communities can be difficult to detect (Forup and Memmott 2005). Spatial and temporal niche partitioning behaviours in response to fluctuating competitive pressure facilitate coexistence among insect groups with dietary overlap, meaning that resource competition is often likely to be a key, but masked, factor in observed flower choice and foraging behaviour (Balfour et al. 2015b). Exclusion experiments in which dominant competitors are removed from flowers are a valuable way to reveal these underlying patterns of competition between insect groups (Balfour et al. 2015b). These can help to improve our understanding of the role of competition in existing pollinator communities, which can in turn help to inform conservation practice for bees and other flower visiting insects, for example by improving provision of floral resources in areas or seasons when they are most needed (Garibaldi et al. 2014; Couvillon et al. 2014; Wignall et al. 2020).
In this study, we used established exclusion methods to investigate whether honeybees and bumblebees compete with each other and with other flower-visiting insects for a common and abundant wildflower, bramble (Rubus fruticosus L. agg.), which produces large quantities of nectar and pollen and is a major food source for many insects (Taylor 2005; Falk and Lewington 2015; Wignall et al. 2020b). We chose to study R. fruticosus as it is common throughout the UK, it has a long flowering season and its flowers are visited by a wide range of insects. Collectively, these factors mean that bramble was a useful and ecologically important species with which to investigate competition among flower visiting species, and that our findings would be of broad relevance.
We established four bee-exclusion treatments: no bees excluded (Control), honeybees excluded (HBE), bumblebees excluded (BBE) and both honey- and bumblebees excluded (HB&BBE) on patches of wild-growing R. fruticosus in two field trials in Sussex, southeast England. We investigated how exclusions affected nectar availability in the bramble flowers, and flower visitation by non-excluded Apis or Bombus bees, solitary bees, hoverflies and other foraging insects. We incorporated landscape-level context by measuring local floral resource availability within a 500 m radius of each study site.
Methods
Study sites
Two eight-day bee-exclusion trials were carried out on the outskirts of Brighton, a city in southeast England, UK. The first trial (20.06.2019–02.07.2019) studied bramble growing in a semi-rural location, in a field of unimproved grassland occasionally used for sheep grazing in the South Downs, 1 km southeast of the University of Sussex campus (hereafter Site 1; 50° 854374‵ N, −0° 09413480‵ W). The second trial (04.07.2019–13.07.2019) was carried out in an area of urban greenfield with bramble growing in hedges, next to residential housing 200 m north of Brighton Marina (hereafter Site 2; 50° 815178‵ N, −0° 102075‵ W). In each location, bramble plants were abundant and in full bloom in hedges and stand-alone patches from c. 0.5 to 4 m above ground level. Although there were no records of the number of managed or feral colonies in the study region, it was assumed that honeybee numbers would be relatively consistent between the sites since there are many active beekeepers in East Sussex and density of honeybees is high in the region (Wignall et al. 2020b). Additionally, the study sites were just over 4 km apart meaning that the number of colonies between the two sites would not vary too greatly. The number of honeybees visiting control patches was similar between sites which supports this assumption (Results).
Exclusions and experimental design
In each eight-day trial, four patches of bramble were designated and rotated through four bee-exclusion treatments: CON: control, no bees excluded; HBE: honeybees excluded; BBE: bumblebees excluded; HB&BBE: both honeybees and bumblebees excluded. This allowed us to investigate the effects of competition exerted by Apis and Bombus separately and in tandem. Using established methods, bees were continually excluded by tapping them gently with a bamboo cane. This method is very effective in reducing the excluded bee type to close to zero (Balfour et al. 2015b; Wignall et al. 2020); in this study, numbers of excluded bee types recorded foraging on HBE, BBE and HB&BBE were between 1.4–13.1% of the total insects per treatment at Site 1 and 0.5–4.4% at Site 2 (Online Resources 1 and 2). Due to this experimental design, it was not possible to record data blind to the patch treatment.
We chose patches of bramble in full bloom, as similar in flower density, patch size and exposure to sun and wind as possible. Each was approximately 2 × 4 m wide and was trimmed as necessary to consistent sizes and numbers of flowers. Patches were always between 10 and 200 m apart to ensure they were distinct. Treatments were allocated randomly to the four patches and rotated after two days of data collection, so that each patch was used for each of the four exclusion treatments, to control for any possible differences between patches, including microclimate effects or presence of bee or wasp nests in the close vicinity. Each two-day rotation was on two consecutive days so that the effect of exclusions on day one would directly influence day two. The distance between patches meant that excluded honey- and bumblebees, which both commonly forage to distances greater than 200 m (Seeley 1995; Walther-Hellwig and Frankl 2000), would feasibly be able to move to nearby treatment patches from which they had not been removed (CON or BBE/HBE). However, the likelihood that the number of bees on other treatment patches could be inflated by excluded individuals is minimal since flowering bramble plants were abundant at both sites, with treatment patches estimated to comprise < 5% of the total bramble within a 50 m radius. Bramble was also common in the wider area (Online Resource 3).
Data were collected only on days with good insect foraging conditions: > 16 °C, low wind, and no rain. Bees were excluded from 09:00 to 18:00, and counts of all insects foraging on the bramble flowers on each treatment patch were made every half hour from 10:00 to 17:00, the most active foraging hours (Garbuzov and Ratnieks 2014). This allowed treatments to be established for one hour in the morning before data collection started and continued for an hour after the counts ended in order to maintain the exclusion treatments. Counts were ended at 17:00 as by this time insect numbers generally had dropped significantly and some patches were in shade. Since the duration of time spent foraging in one patch of flowers is rarely longer than a few minutes (except for beetles which were not included in this study), 30 min was considered ample time to prevent counting the same insect twice. If an individual revisited the patches after 30 min and was counted again, this was considered to be an independent foraging decision, showing a genuine preference rather than an individual simply persisting in the same patch in a single visit (following Garbuzov and Ratnieks 2014).
Insects were grouped to taxa as follows: honeybees (Apis mellifera), bumblebees (Bombus spp.), solitary bees (non-Apis/Bombus bees), hoverflies, non-syrphid dipteran flies, butterflies and moths and wasps. Where possible, bees, hoverflies, butterflies, moths and wasps were also recorded to species on the wing as they were counted. Bombus terrestris (L.) and the B. lucorum complex comprising the cryptic species B. lucorum (L.), B. magnus Vogt and B. cryptarum (Fabricius) (McKendrick et al. 2017), could not be reliably separated, so were grouped and recorded as Bombus terrestris/lucorum agg. (Fussell and Corbet 1992). Any insects that could not be identified on the wing were caught for closer examination in the field. Where it was not possible to identify insects in the field, specimens were taken to the lab to be identified using a microscope. For Lasioglossum bees, which were numerous at the second site and for the most part have microscopic identifying features, a representative sample was taken as specimens to minimise destructive sampling. We did not attempt to identify non-syrphid Diptera since these would need an expert dipterologist for accurate identification. These were not numerous, only 0.0–2.8% of control patch insects (see Results) and were recorded in four body size categories determined by eye (very small, small, medium, large).
Any territorial hoverfly behaviour was noted, and in some instances, we removed patrolling male Eristalis tenax L. hoverflies since these were actively preventing bees, butterflies and other insects from foraging (Wellington and Fitzpatrick 1981) and were therefore interfering with the exclusion treatments.
Nectar standing crop volume (μl) and sugar content
To quantify nectar availability to foraging insects, we measured the standing crop volume and sugar concentration of nectar in ten bramble flowers per treatment on each trial day. We extracted nectar from each flower’s central nectar disc using glass microcapillary tubes (Drummond Microcap 1 μl, 64 mm, 1–000–0010-64 or 0.25 μl, 32 mm, 1–000-00025). The length of nectar drawn into the tube was measured and used to calculate the per-flower nectar volume in microlitres as a proportion of the overall volume of the tube (Corbet 2003). Nectar sugar concentration (% Brix) was measured for each sample with sufficient volume using a handheld refractometer (Bellingham and Stanley™, 0–50% Brix). Any samples with sugar concentration below 10% were assumed to be mostly rain or dew and discarded, and a sample taken from a new flower. Nectar measurements were made between 12:00 and 14:00 each day to reduce time-dependent variation.
Per-flower nectar sugar content (mg/ml) was calculated using nectar concentration and standing crop volume. Concentration values (% Brix) were converted to sucrose content (mg) for each sample using Table 79 in the CRC handbook of chemistry and physics (1971–1972; Weast 1971). Sucrose (mg) was multiplied by standing crop volume (ml) to give the total sugar content per flower in milligrams per millilitre (Bolten et al. 1979).
Measurement of nectar concentration was subject to sampling biases. First, concentration values are bound at 50% Brix due to the 0–50% refractometer used. Actual concentration may have been higher than 50% where this was recorded, meaning that calculated averages for per-flower sugar content may be conservative. Second, concentration could not be measured for nectar samples with small volumes (Site 1: mean standing crop volume of unmeasurable samples = 0.0531 μl, n = 84 of 320 samples; Site 2: mean = 0.0586 μl, n = 186 of 310) due to refractometer sensitivity. Nectar concentration increases at smaller droplet volumes due to a greater rate of evaporation (Corbet 2003) possibly leading to biased exclusion of samples with higher concentrations. Although any effect of this will be minimised to a degree by the very low volume of excluded samples, reported average per-flower nectar sugar content per treatment may be conservative where many samples were excluded, which disproportionately affects those with lower average standing crop volume (Results). Together, these factors limit the accuracy of nectar sugar content data and these should be interpreted with caution.
Local land-use and wildflower diversity
We mapped the local habitat types and wildflower diversity within a 500 m radius of the centre of each site to add resource availability context to our study. Most solitary bees have a maximum foraging range of < 500 m from their nests (Gathmann and Tscharntke 2002), while average foraging ranges tend to be far smaller than the maximum (Zurbuchen et al. 2010b). Bumblebees also often forage within 500 m of their nest, although they are capable of longer flights (Walther-Hellwig and Frankl 2000). Honeybees are able to forage much longer distances from the hive, up to c. 10–12 km, but normally forage at much lower distances (Seeley 1995; Couvillon et al. 2014). This distance, therefore, gives a meaningful measure of the local resource availability for bees, particularly solitary bees and bumblebees.
We used QGIS 3 (version 3.0.3 Girona) to manually categorise each land-use type within the 500 m radius and to quantify the total area of each. Using this, we then determined two approximate measures of the local resource availability for flower-visiting insects per site. First, the total ‘flowers possible’ surface area, which included any greenspace or habitat where flowers could grow, was compared to the area of impermeable surfaces and water. Within the ‘flowers possible’ area, we then determined the ‘flowers available’ area per site, after removing arable fields, golf courses and sports pitches following site visits to verify that flowers were absent or at extremely low densities in these sub-areas.
Within the ‘flowers available’ area, we calculated the proportion of each land-use type that could be surveyed, excluding areas such as cliff face and residential gardens that we were unable to access. We then used 200 m2 belt transects to measure the presence, abundance and diversity of wildflowers in bloom within each surveyable type (Balfour et al. 2015a). For each site, we used the same number of transects in each type rather than stratifying by land-use area, so that linear habitats such as field margins were sampled with an equal effort since these can be important resources for pollinators (Balfour et al. 2015a) despite being a smaller total area respectively. We completed eight transects per surveyable habitat type at Site 1 and four at Site 2, since the surveyable ‘flowers available’ area at the first site (549,542 m2) was much greater than that of the second site (62,993 m2).
To measure species richness per habitat type, we recorded the presence of any wildflower species found within 1 m on either side of a 100 m transect. At field margins and road verges, transects were 200 m and flowers recorded within 1 m on a single side, so that transects were always 200 m2 in total. To quantify floral abundance (petal area) and diversity, we placed five 1 × 1 m quadrats to alternate sides of the transect at 20 m intervals (or 40 m intervals in the 200 m transects). We recorded the number of floral ‘units’ (flowers, capituli or umbels) of each wildflower species within the quadrat, and later standardised these using wildflower guides (Streeter et al. 2009; Rose 1981) to give a measure of petal area for each unit, a relevant measure of floral abundance for foraging insects (Balfour et al. 2015a). All surveys for each site were completed within one week of trial completion to ensure a relevant measure of current flower availability in the area.
Statistical analysis
Data from each site were analyzed separately to explore the effects of bee exclusion treatments on insect visitation and nectar characteristics, since the large difference in number of insects between sites (Results) masked the effects of treatment when site was included as a predictor variable. Data presented and analysed include both exclusion days of each rotation to maximise statistical power, meaning reported effects will be conservative since insect response to exclusion takes approximately one day to reach its full effect (Balfour et al. 2015b), which also affects nectar volume. All statistics were calculated using R (version 4.0.0). Significance was taken as P < 0.05.
Insect count data included half-hourly counts between 10:00 and 17:00 on each exclusion day (n = 15 counts per day per treatment, 8 days per trial). For each site, the effects of exclusion treatments on counts of each insect group were analyzed using zero-inflated generalised linear mixed models (GLMM) with glmmTMB to account for an excess of zeros in the count data (Brooks et al. 2017). Fixed effects were treatment (CON, HBE, BBE and HB&BBE) and insect group (honeybees, bumblebees, solitary bees, hoverflies and other insects (comprising butterflies/moths, non-syrphid dipteran flies and wasps, grouped as ‘other insects’ due to small sample sizes, see Results)), with an interaction term between these variables. Rotation and patch were included as crossed random effects. Estimated marginal means and post hoc pairwise comparisons for the effect of treatment within each insect group were calculated using emmeans (Lenth 2019).
At the first site, there were very low numbers of solitary bees (between 0.13 and 0.31 bees per count per treatment on average, 0 bees in 81.1% of counts; Online Resource 1); therefore, this group was included as ‘solitary bees’ without subsetting for optimal model stability and reliability of results. At the second site, there were larger numbers of solitary bees (between 1.12 and 3.22 bees per count per treatment on average; Online Resource 2). Therefore, to explore whether solitary bee foraging response to bee exclusion treatment varied with body size, solitary bees were included as two separate insect groups: larger-bodied solitary bees (forewing > 5.5 mm (Falk and Lewington 2015); Genera: Andrena, Anthophora, Megachile, Osmia) which are similar in size to honey- and bumblebees and are likely to be affected by any changes in floral reward caused by the removal of these bees (Henry and Rodet 2018), and smaller-bodied solitary bees (forewing < 5.5 mm (Falk and Lewington 2015); Genera: Halictus, Hylaeus, Lasioglossum) that have far smaller nectar and pollen requirements (Müller et al. 2006) and are likely to be less affected by any changes in floral reward. Final models were compared to null models using likelihood ratio tests. Scaled residuals were plotted and visually approved for both final models using R package DHARMa (Hartig 2020), with further targeted goodness-of-fit tests for over- and underdispersion between the observed vs simulated residuals.
At the first site, solitary bee diversity was not compared between treatments due to very low numbers of foraging bees meaning that any differences were highly likely to be due to chance (see above and Online Resource 1). At the second site, a standard measure of diversity, the Shannon-Wiener H’ diversity index, was used to compare solitary bee species diversity between treatments:
where p (= n/N) is the proportion of the total sample (N) represented by species i (n). Pielou’s measure of species evenness (J’) was also calculated for each treatment by dividing the Shannon-Wiener H’ index by the natural logarithm of species richness (J’ = H′/lnS).
For each site, one-way analysis of variance (ANOVA) was used to investigate the effect of treatment on (i) nectar standing crop volume (μl) and (ii) sugar content per flower (mg/ml), with Patch and Rotation included as crossed blocking factors. Tukey’s HSD post hoc tests were performed for pairwise comparisons between treatments.
Wildflower diversity and species evenness were calculated for each habitat type using quadrat data and the Shannon-Wiener H’ diversity index and Pielou’s J’ measure of evenness, as described for solitary bees. Individual transects were combined to calculate H’ for each habitat type.
Results
Bee responses to exclusions
Site 1
At Site 1, in the more rural location, numbers of insects observed foraging on the bramble patches were lower (total insects counted = 2070, n = 471 counts) than at Site 2 (total insects counted = 2910, n = 476 counts), despite many insects seen foraging on other flower species in the area. The most numerous insects on CON patches were honeybees (52.8%) and bumblebees (20.1%; 6 species across all treatments). There were very few solitary bees (2.4%; 13 species). Among these were two cuckoo species of solitary bee, Nomada ruficornis L. and Sphecodes monilicornis Kirby, which were only 3% of recorded solitary bee individuals. Hoverflies were the most abundant non-bee insects (18.4%; 17 species). Other insects were not numerous: other non-syrphid dipteran flies, 2.8%; wasps, 2.1% (4 species) and Lepidoptera, 1.3% (7 species; 6 butterfly, 1 moth, Macroglossum stellatarum L.; Online Resource 1).
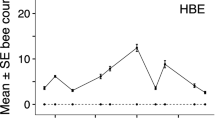
The effect of bee exclusion treatment on insect foraging response varied between insect groups, with a significant interaction between these predictor variables (treatment x insect group, χ2(12) = 509.541, P < 0.001). Bumblebees were significantly affected by the exclusion of honeybees, with an 89% increase in the estimated marginal mean (EMM) count of bumblebees on HBE (EMM ± 1SE, 2.36 ± 0.25) vs CON (1.25 ± 0.14; P < 0.001 in post hoc pairwise comparisons between treatments). Within Bombus, there were increases in 5/6 species (between 36 and 583% increase in raw count data per species; raw data in Online Resource 1). Conversely, bumblebee exclusion did not affect honeybee numbers (P = 1.000). The EMM count of honeybees on BBE treatment patches (2.85 ± 0.25) was 4% lower than CON (2.97 ± 0.25; Table 1; Fig. 1).
Numbers of insects foraging on patches of bramble with four bee exclusion treatments (no bees excluded (CON), honeybees excluded (HBE), bumblebees excluded (BBE) and both honey and bumblebees excluded (HB&BBE)) at Site 1. Estimated marginal means with 95% confidence interval from a zero-inflated generalised linear mixed model are plotted in black. Grey points show the raw count data, jittered horizontally and vertically for clearer visualisation
Solitary bees, hoverflies and other insects (comprising butterflies/moths, non-syrphid dipteran flies and wasps) showed no response to bee exclusion, with all post hoc pairwise comparison between treatments non-significant (P > 0.05; Table 1; Fig. 1).
Site 2
At Site 2, in the more urban location, insect numbers were 40.6% higher overall than at Site 1 (total insects counted = 2910, n = 476 counts). The most numerous insects on CON patches were honeybees (38.3%), followed by bumblebees (36.3%; 5 species across all treatments) and solitary bees (16.4%; 16 species). Within the solitary bees, larger-bodied bees made up 6.7% of the total insects on CON, and smaller-bodied 9.8%. Hoverflies were the most abundant non-bee insects (8.0%; 14 species), followed by wasps, 0.7% (2 species) and Lepidoptera (butterflies only; 4 species), 0.2%. Zero non-syrphid dipteran flies were counted on CON patches (Online Resource 2).
The effect of bee exclusion treatment on insect foraging response varied between insect groups, with a significant interaction between these predictor variables (treatment x insect group, χ2(15) = 578.560, P < 0.001). Honeybees and bumblebees were significantly affected by the exclusion of the ‘opposite’ bee, with a 46% increase in the estimated marginal mean (EMM) count of honeybees on BBE (EMM ± 1SE, 3.75 ± 0.38) vs CON (2.57 ± 0.27), and 47% increase in bumblebees on HBE (3.56 ± 0.36) vs CON (2.43 ± 0.26; P < 0.001 in post hoc pairwise comparisons; Table 2, Fig. 2).
Numbers of insects foraging on patches of bramble with four bee exclusion treatments (no bees excluded (CON), honeybees excluded (HBE), bumblebees excluded (BBE) and both honey and bumblebees excluded (HB&BBE)) at Site 2. Estimated marginal means with 95% confidence interval from a zero-inflated generalised linear mixed model are plotted in black. Grey points show the raw count data, jittered horizontally and vertically to reduce overlap for clearer visualisation
Larger-bodied solitary bees increased significantly on each exclusion treatment (HBE, BBE and HB&BBE; P < 0.001) relative to CON (0.45 ± 0.07 bees), with the largest number of bees on HB&BBE (2.46 ± 0.28), an increase of 447%. Smaller-bodied solitary bees and hoverflies showed no response to bee exclusion, with all post hoc pairwise comparison between treatments non-significant (P > 0.05; Table 2, Fig. 2). At the species level, nine out of 12 larger-bodied solitary bee species were more numerous on all three exclusion treatment patches compared to CON (raw data in Online Resource 2).
The number of other insects (comprising butterflies/moths, non-syrphid dipteran flies and wasps) was very small on all exclusion treatments. There was a slight increase in EMM on each treatment relative to CON, with the largest on HB&BBE, 0.35 ± 0.07 insects, which was significantly higher than CON (0.07 ± 0.02, P < 0.05). All other post hoc pairwise tests were non-significant. Hoverflies were also not numerous and numbers were similar across treatments (between 0.51 ± 0.08 (HBE) and 0.70 ± 0.11 (HB&BBE); P > 0.05; Table 2, Fig. 2).
There were slight differences in solitary bee species diversity between treatments at Site 2. Shannon Wiener H’ diversity index and species richness (S) were similar on BBE (H’ = 1.99; S = 15), HB&BBE (H’ = 1.96; S = 13) and HBE (H’ = 1.91; S = 14), and lowest on CON (H’ = 1.70; S = 10). Species evenness (J’) was similar between treatments (J’: CON, 0.74; HBE, 0.72; BBE, 0.74; HB&BBE, 0.76).
Nectar standing crop volume (μl) and per-flower sugar content (mg/ml)
Treatment significantly affected nectar standing crop volume at Site 1 (F(3) = 11.225, P < 0.001). Standing crop volume was significantly higher in HB&BBE patch flowers (mean ± SD, 1.76 ± 1.88 μl) compared to all other treatments (CON 0.647 ± 0.871 μl, + 172%; HBE 0.876 ± 1.30 μl, + 101%; BBE 0.792 ± 1.21 μl, + 122%; n = 80 flowers per treatment; P < 0.001). Standing crop volume was not significantly different between any other treatments (P > 0.05; Fig. 3a). At Site 2, mean nectar standing crop volumes were between 80 and 87% lower in each treatment than at Site 1, although the trends were similar (Fig. 3). Treatment also significantly affected nectar standing crop volume at Site 2 (F(3) = 17.679, P < 0.001). Nectar volume was significantly higher in HB&BBE patch flowers (0.303 ± 0.258 μl, n = 70 flowers) compared to all other treatments (CON 0.128 ± 0.166 μl (n = 80), + 137%; HBE 0.135 ± 0.176 μl (n = 80), + 124%; BBE 0.101 ± 0.134 μl (n = 80), + 200%; P < 0.001). Standing crop volume was not different between other treatments (P > 0.05; Fig. 3b). Nectar concentration was recorded as 50% (Brix) for nine standing crop samples at Site 1 (CON 1 sample, HBE 1, BBE 3, HB&BBE 4) and 56 samples at Site 2 (CON 9, HBE 5, BBE 10, HB&BBE 32). Actual concentration may have been > 50% for these samples, meaning that calculated per-flower nectar content values may be conservative (see the “Nectar standing crop volume (μl) and sugar content” section).
Standing crop volumes (μl) of nectar across the four bee exclusion treatments (no bees excluded (CON), honeybees excluded (HBE), bumblebees excluded (BBE) and both honey and bumblebees excluded (HB&BBE)) at Site 1 (a) and Site 2 (b). The plots show the volume of nectar collected from ten flowers per patch treatment per day over the eight-day trial (CON, HBE, BBE: n = 80 flowers in total per treatment; HB&BBE, n = 70). Horizontal lines show the median, boxplot limits are the 25th and 75th percentiles, whiskers are 1.5 times the interquartile range and points outside whiskers represent outliers. Crosses within plots represent the mean nectar volume per treatment (μl). Different superscript letters above boxes (A, B) denote significant difference between treatments at P < 0.05 according to ANOVA with Tukey’s post hoc pairwise comparisons
Per-flower sugar content could be calculated for 73.75% of extracted nectar samples at Site 1; 84 of 320 samples had standing crop volumes that were too low (mean = 0.053 μl) to measure concentration (CON 22 samples, HBE 25, BBE 28, HB&BBE 8).
Nectar sugar content (calculated using available concentration data) was highest in HB&BBE patch flowers (0.835 ± 0.834 mg/ml, n = 72). This was significantly higher than CON (0.266 ± 0.231 mg/ml, + 214%, n = 58; P < 0.001), BBE (0.371 ± 0.469 mg/ml, + 125%, n = 52; P < 0.001) and HBE (0.487 ± 0.588 mg/ml, + 72%, n = 61; P = 0.005). Sugar content per flower was not significantly different between any other treatment pairs (P > 0.05).
At Site 2, per-flower nectar sugar content could be calculated for only 40% of samples; 186 of 310 sample volumes were too low (mean = 0.0586 μl) to measure concentration (CON 51 samples, HBE 56, BBE 57, HB&BBE 22). Nectar sugar content (calculated using available concentration data) was highest in HB&BBE patch flowers (0.240 ± 0.164 mg/ml, n = 58); this was significantly higher than CON (0.143 ± 0.101 mg/ml, + 68%, n = 29; P = 0.009) and BBE (0.140 ± 0.079 mg/ml, + 71%, n = 23; P = 0.013), and non-significantly, 40%, higher than HBE (0.171 ± 0.103 mg/ml, n = 24; P = 0.141). Sugar content per flower was not significantly different between any other treatment pairs (P > 0.05; see Methods: Nectar standing crop volume (μl) and sugar content regarding caution in interpreting nectar sugar content data).
Local land-use and wildflower diversity
A high proportion of the 500 m radius area (= total area 785,398 m2) was successfully categorised into land-use types in each site (Site 1: 783711 m2, 99.7%; Site 2: 768107.5 m2, 97.8%). Within these areas, Site 1 had a much greater proportion of ‘flowers possible’ area, comprising arable fields, broad-leaved woodland, field margins, residential gardens, sports pitches and unimproved grassland (87.2%) versus 12.8% impermeable surfaces. Site 2 had a much smaller proportion of ‘flowers possible’ area, including broad-leaved woodland, cliff face, cliff top verges, field margins, golf course, nature reserve land, residential gardens, sports pitches, urban greenfield areas and other urban greenspace (38.1%) versus 61.9% impermeable surfaces or water.
The ‘flowers available’ area per site, including land-use types verified to have flowers present, also comprised a much higher proportion of the overall habitat at Site 1 (74.7%; 585,216/783711 m2) compared to Site 2 (22.9%; 176,143.5/768107.5 m2; Fig. 4). Detailed information of petal area, a measure of floral abundance, and diversity indices from transect and quadrat data for each surveyed ‘flowers available’ habitat type is in Online Resource 3.
Maps showing the habitat surrounding Site 1 (a) and Site 2 (b). Black circles define the 500 m radius area surrounding the site centre, which is shown as a yellow square. White scale bars on the bottom left of maps show 100 m. Corresponding pie charts show the proportion of habitat within the 500 m radius area that was found to have ‘flowers available’ (green; Site 1 = 74.7%, Site 2 = 22.9%), compared to the proportion of area made up of impermeable surface and/or with flowers absent (grey; Site 1 = 25.3%, Site 2 = 77.1%). Maps were created using QGIS with ESRI “Satellite” base map
Discussion
Our results show that visitation to wild-growing bramble flowers by honeybees and bumblebees decreases per-flower nectar volume and can reduce visits by other foragers, suggesting that Apis and Bombus displace other insects through exploitative competition. Our study is the first, to our knowledge, to demonstrate reciprocal competition between Apis and Bombus on one flower species. Another notable finding was the strong displacement of non-Apis/Bombus bees (referred to here as ‘solitary bees’) in one of two study sites, where local flower availability was somewhat limited and competition for nectar and pollen was likely to be high. The observed effects on hoverflies and other insects (butterflies, wasps and non-syrphid dipteran flies) were inconclusive.
Reciprocal competition between Apis and Bombus
The separate removal of Apis or Bombus from bramble patches revealed reciprocal competition. Bumblebee numbers increased following honeybee removal both at Site 2 (47%) and at Site 1 (89%) compared to control patches, showing clear ecological release from competition. This was consistent for each (n = 5/5) bumblebee species at Site 2, and all apart from B. vestalis Geoffroy (n = 5/6) at Site 1. Honeybee numbers increased following bumblebee exclusion at Site 2 (46%), but did not show any release from competition at Site 1 (− 4%; Figs. 1 and 2).
Honey- and bumblebees are often the most abundant foragers on R. fruticosus and both rapidly extract nectar from the open-structured Rosaceae-type flowers (Couvillon et al. 2015; Wignall et al. 2020). The removal of either group should, therefore, cause an increase in nectar availability, as well as pollen. Since both honeybees (Duffield et al. 1993; Balfour et al. 2015b) and bumblebees (Heinrich 1976) choose flowers with a higher reward, this is likely to explain the increase in the number of Apis and Bombus on patches where the ‘opposite’ bee had been removed (Fig. 1). Although we did not record an increase in per-flower nectar standing crop volume or sugar content in HBE or BBE patches compared to controls at either site, this is likely to be due to the quick depletion of any nectar by the ‘other’ (non-excluded) bee to levels similar to control patches (Fig. 3).
The anomalous result at Site 1, where honeybee numbers did not increase on BBE treatment patches compared to control patches, may be due to the low proportion of bumblebees visiting the bramble flowers (20% of all insects on control patches, compared to 53% honeybees). At this site, removing bumblebees would have had only a small effect on nectar availability for honeybees, which may explain why honeybee numbers did not increase although absolute numbers remained high (Online Resource 1). In contrast, the significant increase in bumblebee numbers on HBE patches at this site showed that bumblebees were released from competitive pressure exerted by honeybees.
These patterns are in line with the ideal free distribution model, in which the number of foragers exploiting a resource is proportional to the rate of reward production in the patch, such that reward gain per forager is equal across resource patches (Fretwell and Lucas 1970).
Solitary bees
Solitary bee response to Apis and Bombus exclusion varied between sites. At Site 2, a substantial and significant increase in the number of larger-bodied solitary bee visits to bramble flowers following removal of honeybees or bumblebees, and especially both, is strong evidence for ecological release from competition (Fig. 2). In comparison, at Site 1, there was no evidence of competition exerted by eusocial bees, with no measurable response likely due to the very low abundance of solitary bees on all treatment patches (Fig. 1). This between-site difference may be due to local flower availability, which, as discussed in the Results, was substantially lower at Site 2 (Fig. 4).
At Site 2, when both honey- and bumblebees were excluded (HB&BBE), there was a very large, 447%, and significant increase in larger-bodied solitary bee visits to bramble flowers compared to control patches (Fig. 2). This may be partly explained by the corresponding 137% rise in nectar availability in HB&BBE patch flowers (Fig. 3b), and 68% measured increase in per-flower sugar content (although reported sugar content data may be conservative, see Methods). Like eusocial bees, solitary bees are sensitive to changes in nectar reward which is a key factor in flower choice and foraging patterns (Howell and Alarcón 2007; Mallinger and Prasifka 2017). However, other factors may also have contributed, including, importantly, pollen availability since this is also a major factor driving solitary bee foraging behaviour (Tepedino and Parker 1982; Williams and Tepedino 2003) as females must provision nest cells with large quantities of pollen to rear developing larvae (Müller et al. 2006). The physical presence of honey- and bumblebees is another possible factor. It would be interesting to investigate these in further studies, for example by measuring pollen availability in each treatment patch, or by decoupling bee exclusions from changes in nectar and pollen reward by experimentally adjusting these to determine if the solitary bees respond to competitors directly or via their effect on resource amounts.
Our results also suggest that body size may affect the extent to which exploitative competition affects solitary bees, which is consistent with previous findings (Torné-Noguera et al. 2016; Henry and Rodet 2018). At Site 2, the increase in solitary bee visits to HB&BBE compared to control patches was substantial, 447%, and significant for larger-bodied solitary bees (forewing > 5.5 mm; Genera: Andrena, Anthophora, Megachile, Osmia). In contrast, the number of smaller-bodied bees (forewing < 5.5 mm (Falk and Lewington 2015); Genera: Hylaeus, Halictus, Lasioglossum), was similar between treatment patches; a 35% increase on HB&BBE patches compared to control patches was not significant (Table 2, Fig. 2). Smaller bees have correspondingly smaller requirements for nectar and pollen (Müller et al. 2006) and may therefore be less affected by the depletion of these resources by honey- and bumblebees. The effect of body size may particularly relate to pollen availability, which is likely to be affected by pollen removal by honey- and bumblebees, since larger-bodied bees require large quantities to rear larvae (Müller et al. 2006). It is also possible that the very small foraging ranges of the smaller-bodied genera, particularly Lasioglossum species (Wright et al. 2015), may have prevented them from discovering the patches where bees were excluded.
Separate exclusion of honeybees or bumblebees (HBE and BBE treatments) at Site 2 also caused an increase in the number of larger-bodied solitary bee foragers (Fig. 2). This response was smaller than when both groups were removed (HB&BBE), which is likely to be due to the increase in Bombus or Apis visitation to HBE and BBE patches in response to the exclusion of the ‘opposite’ bee group (Fig. 1b), which, as well as possible un-measured factors such as pollen depletion or physical presence, depleted per-flower nectar volume to levels similar to control patches (Fig. 3b). Nevertheless, this shows that the removal of either group singly can reduce competitive pressure to a degree that allows solitary bees to forage on flowers from which they had previously been displaced. Possible longer-term effects of competitive displacement are discussed in the “Possible fitness effects of competitive displacement” section.
In contrast to Site 2, at Site 1, there were consistently very few solitary bees visiting bramble flowers in all treatment patches (< 0.35 per count per treatment on average, only 2.4% of all insects on control patches). This was despite substantial nectar standing crop volume and sugar content in the flowers in each patch, a 172% increase in per-flower nectar volume in HB&BBE patches compared to CON (Fig. 3a), and observations of many solitary bees foraging on other nearby flowers. There were small increases in solitary bee visitation to exclusion treatments compared to controls but no significant differences between treatments. Overall, exploitative competition between bees for bramble flowers was much lower or non-existent in this location, which may be a result of high local flower availability (see Local floral resource availability) although this inference is limited due to the two-site comparison in this study (see Limitations and further research).
Hoverflies and other non-bee insects (butterflies, wasps and non-syrphid dipteran flies)
Previous research has found that experimentally enhanced honeybee densities spatially displaced wild non-bee insects including hoverflies and non-syrphid flies in fields of flowering oilseed rape (Lindström et al. 2016). However, very little research has so far investigated the effect of resource depletion by Apis and Bombus on non-bee insects.
In this study, the numbers of Lepidoptera, wasps and non-syrphid dipteran flies at both sites were small and there was no clear response to bee exclusion. Hoverflies were more numerous, but showed a mixed response to bee removals, also with no clear effect of exclusions. It is also not clear from our data whether hoverfly flower visitation was linked to nectar reward. At Site 1, average hoverfly numbers were lowest on the two patch treatments with the lowest per-flower nectar standing crop volume and sugar content (CON and BBE). However, hoverflies were most numerous on HBE patches where per-flower nectar volume and sugar content were almost half that of HB&BBE patches on average. At Site 2, the number of hoverflies visiting each treatment patch was highly similar between treatments, meaning that patch visitation also cannot be meaningfully linked to nectar reward.
Overall, this inconclusive result may be due to hoverflies’ different life history strategy since, unlike bees, they do not rear young in a central nest. As such, they must only meet their individual energy requirements and do not have to provision a nest or feed larvae, with broader requirements including suitable oviposition sites and larval food material. This is also the case for Lepidoptera and non-syrphid dipteran flies. While foraging behaviour in these groups is therefore very different to bees, with requirements other than maximizing efficient nectar and pollen collection, it is not clear how they are affected by immediate local resource depletion, or whether they may be more resilient to Apis/Bombus-mediated exploitative competition.
Local floral resource availability
Local availability of floral resources was markedly greater at Site 1 than at Site 2 (Fig. 4). At Site 1, 75% of the land within 500 m was verified to have wildflowers growing and in bloom (‘flowers available’). Almost 60% of this was unimproved grassland immediately adjacent to the study field, which was the most flower-abundant of the surveyed habitat types at this site (Online Resource 3).
In contrast, only 23% of the land area surrounding Site 2 was found to have ‘flowers available’. Flower-rich habitat was patchily distributed at this location (Fig. 4), and 62% of the land within a 500 m radius was made up of buildings, roads, other impermeable surfaces and seawater. A further 15% was amenity grassland verified to have very few or zero flowers present. Overall, local availability of flowers for foraging insects was limited, particularly for those with small foraging ranges such as solitary bees which rarely fly further than 500 m from their nests (Gathmann and Tscharntke 2002).
This substantial difference in surrounding land use within 500 m of the study site may explain the inconsistent solitary bee response to Apis/Bombus exclusion between Sites 1 (no competition evident) and 2 (evidence of strong competition), since lower flower availability is likely to increase the strength of competition among flower-visiting insects, and vice versa. For example, a study by Thomson (2016) found that competition between bees was stronger in drought years when the availability of preferred flower species was reduced. Previous research has also suggested that exploitative competition between bees is weak when local per-insect nectar availability is high, compared to strong competition when nectar availability is low (Wignall et al. 2020). However, further research would be needed to confirm whether the degree of exploitative competition between bees on bramble flowers can be explained by local flower availability.
Possible fitness effects of competitive displacement
We were not able to measure population-level effects of competitive exclusion from bramble flowers in this study. However, it is possible that in landscapes where floral resources are limited or bramble provides a major source of nectar and pollen, long-term displacement of bees from this abundant resource may negatively affect their reproductive output.
Competitive displacement from preferred flowers causes honeybee (Walther-Hellwig et al. 2006), bumblebee (Walther-Hellwig et al. 2006; Thomson 2016) and solitary bee (Hudewenz and Klein 2015; Villanueva-Gutiérrez et al. 2015) species to move to alternative sources of forage, which may be of lower quality, lower quantity, and/or further away causing longer foraging distances to find nectar and pollen. This can have a fitness cost, including reduced offspring production. For example, foraging over longer distances has been shown to reduce the number of brood cells provisioned per unit of time in two species of solitary bee (Zurbuchen et al. 2010a).
Solitary bee species, many of which have undergone declines in abundance and distribution in recent decades (Falk and Lewington 2015), may be particularly vulnerable to the effects of competitive displacement as they have smaller foraging distances often of < 500 m from their nests (Gathmann and Tscharntke 2002; Zurbuchen et al. 2010b) compared to the larger foraging ranges of honeybees (Couvillon et al. 2014) and many bumblebee species (Walther-Hellwig and Frankl 2000). As a result, they have a comparatively reduced capacity to find alternative sources of forage, especially where these are limited. This is potentially concerning since solitary bees may simultaneously be particularly susceptible to the process of competitive displacement by eusocial bees, as shown in our results.
Limitations and further research
In this study, we completed field trials in two locations. However, it would be beneficial to carry out trials using patches of bramble in more locations on a gradient of local floral abundance, in order to support our findings as well as to confirm whether the strength of Apis/Bombus-mediated exploitative competition is affected by floral resource availability (and to what extent). Further research is also needed to understand the extent of this phenomenon in flower-visiting insect communities on other wildflower and crop species, to extend the breadth of the findings and since pollinator abundance and diversity is often vital for effective pollination (Garibaldi et al. 2013).The interacting effects of landscape-scale resource availability and the abundance of managed pollinators (currently mostly honeybees) on other pollinator species deserves further attention particularly as large-scale anthropogenic changes continue to reduce net floral availability for pollinators, including agricultural intensification (Ollerton et al. 2014; Senapathi et al. 2017) and urbanisation (McKinney 2006). Lastly, in this study, we were not able to measure population-level effects of competitive displacement by Apis and Bombus on each other and other insect groups, which is an important next step.
Conclusions
Overall, our findings reveal multiple underlying competitive interactions shaping a wild pollinator community. Exploitative competition exerted by bumblebees and particularly honeybees may be widespread in pollinator communities, with particularly strong effects where floral resources are limited. Per-insect resource availability is critical for their survival, health and reproductive success (Balfour et al. 2018), including through buffering against other stressors such as pathogens (Brown et al. 2000; Goulson et al. 2015). Therefore, understanding patterns of competition and displacement is necessary for pollinator conservation, particularly for vulnerable or threatened species. This should be taken into account in landscape management, both to inform honeybee stocking densities and, importantly, to maximise provision of floral resources when and where they are most needed.
Data availability
Data will be made available from the Dryad Digital Repository on acceptance.
References
Amarasekare P (2003) Competitive coexistence in spatially structured environments: a synthesis. Ecol Lett 6:1109–1122. https://doi.org/10.1046/j.1461-0248.2003.00530.x
Baldock KCR, Goddard MA, Hicks DM, Kunin WE, Mitschunas N, Osgathorpe LM, Potts SG, Robertson KM, Scott AV, Stone GN, Vaughan IP, Memmott J (2015) Where is the UK’s pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proc R Soc B 282:20142849. https://doi.org/10.1098/rspb.2014.2849
Baldock KCR, Goddard MA, Hicks DM, Kunin WE, Mitschunas N, Morse H, Osgathorpe LM, Potts SG, Robertson KM, Scott AV, Staniczenko PPA, Stone GN, Vaughan IP, Memmott J (2019) A systems approach reveals urban pollinator hotspots and conservation opportunities. Nat Ecol Evol 3:363–373. https://doi.org/10.1038/s41559-018-0769-y
Balfour NJ, Fensome KA, Samuelson EW, Ratnieks FLW (2015a) Following the dance: ground survey of flowers and flower-visiting insects in a summer foraging hotspot identified via honeybee waggle dance decoding. Agric Ecosyst Environ 213:265–271. https://doi.org/10.1016/j.agee.2015.08.007
Balfour NJ, Gandy S, Ratnieks FLW (2015b) Exploitative competition alters bee foraging and flower choice. Behav Ecol Sociobiol 69:1731–1738. https://doi.org/10.1007/s00265-015-1985-y
Balfour NJ, Ollerton J, Castellanos MC, Ratnieks FLW (2018) British phenological records indicate high diversity and extinction rates among late-summer-flying pollinators. Biol Conserv 222:278–283. https://doi.org/10.1016/j.biocon.2018.04.028
Bolten AB, Feinsinger P, Baker HG, Baker I (1979) On the calculation of sugar concentration in flower nectar. Oecologia 41:301–304. https://doi.org/10.1007/BF00377434
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400. https://doi.org/10.32614/RJ-2017-066
Brown MJF, Loosli R, Schmid-Hempel P (2000) Condition-dependent expression of virulence in a trypanosome infecting bumblebees. Oikos 91:421–427. https://doi.org/10.1034/j.1600-0706.2000.910302.x
Cane JH, Tepedino VJ (2016) Gauging the effect of honey bee pollen collection on native bee communities. Conserv Lett 10:205–210. https://doi.org/10.1111/conl.12263
Corbet S (2003) Nectar sugar content: estimating standing crop and secretion rate in the field. Apidologie 34:1–10. https://doi.org/10.1051/apido:2002049
Couvillon MJ, Schürch R, Ratnieks FLW (2014) Waggle dance distances as integrative indicators of seasonal foraging challenges. PLoS One 9:e93495. https://doi.org/10.1371/journal.pone.0093495
Couvillon MJ, Walter CM, Blows EM, Czaczkes TJ, Alton KL, Ratnieks FLW (2015) Busy bees: variation in insect flower-visiting rates across multiple plant species. Psyche J Entomol:e134630. https://doi.org/10.1155/2015/134630
Duffield GE, Gibson RC, Gilhooly PM, Hesse AJ, Inkley CR, Gilbert FS, Barnard CJ (1993) Choice of flowers by foraging honey bees (Apis mellifera): possible morphological cues. Ecol Entomol 18:191–197. https://doi.org/10.1111/j.1365-2311.1993.tb01089.x
Dupont YL, Hansen DM, Valido A, Olesen JM (2004) Impact of introduced honey bees on native pollination interactions of the endemic Echium wildpretii (Boraginaceae) on Tenerife, Canary Islands. Biol Conserv 118:301–311. https://doi.org/10.1016/j.biocon.2003.09.010
Falk SJ, Lewington R (2015) Field guide to the bees of Great Britain and Ireland (field guides). British Wildlife Publishing, Totnes
Forup ML, Memmott J (2005) The relationship between the abundances of bumblebees and honeybees in a native habitat. Ecol Entomol 30:47–57. https://doi.org/10.1111/j.0307-6946.2005.00660.x
Fretwell SD, Lucas HL (1970) On territorial behaviour and other factors influencing habitat distribution in birds. Acta Biotheor 19:16–36. https://doi.org/10.1007/BF01601953
Fussell M, Corbet SA (1992) Flower usage by bumble-bees: a basis for forage plant management. J Appl Ecol, 29:451–465. https://doi.org/10.2307/2404513
Garbuzov M, Ratnieks FLW (2014) Quantifying variation among garden plants in attractiveness to bees and other flower-visiting insects. Funct Ecol 28:364–374. https://doi.org/10.1111/1365-2435.12178
Garibaldi LA, Steffan-Dewenter I, Winfree R, Aizen MA, Bommarco R, Cunningham SA, Kremen C, Carvalheiro LG, Harder LD, Afik O, Bartomeus I, Benjamin F, Boreux V, Cariveau D, Chacoff NP, Dudenhoffer JH, Freitas BM, Ghazoul J, Greenleaf S, Hipolito J, Holzschuh A, Howlett B, Isaacs R, Javorek SK, Kennedy CM, Krewenka KM, Krishnan S, Mandelik Y, Mayfield MM, Motzke I, Munyuli T, Nault BA, Otieno M, Petersen J, Pisanty G, Potts SG, Rader R, Ricketts TH, Rundlof M, Seymour CL, Schuepp C, Szentgyorgyi H, Taki H, Tscharntke T, Vergara CH, Viana BF, Wanger TC, Westphal C, Williams N, Klein AM (2013) Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339:1608–1611. https://doi.org/10.1126/science.1230200
Garibaldi LA, Carvalheiro LG, Leonhardt SD, Aizen MA, Blaauw BR, Isaacs R, Kuhlmann M, Kleijn D, Klein AM, Kremen C, Morandin L, Scheper J, Winfree R (2014) From research to action: enhancing crop yield through wild pollinators. Front Ecol Environ 12:439–447. https://doi.org/10.1890/130330
Garratt MPD, Truslove L, Coston D, Evans R, Moss E, Dodson C, Jenner N, Biesmeijer JC, Potts S (2014) Pollination deficits in UK apple orchards. J Poll Ecol 12:9–14. https://doi.org/10.26786/1920-7603(2014)8
Gathmann A, Tscharntke T (2002) Foraging ranges of solitary bees. J Anim Ecol 71:757–764. https://doi.org/10.1046/j.1365-2656.2002.00641.x
Goulson D, Sparrow KR (2009) Evidence for competition between honeybees and bumblebees; effects on bumblebee worker size. J Insect Conserv 13:177–181. https://doi.org/10.1007/s10841-008-9140-y
Goulson D, Lye GC, Darvill B (2008) Decline and conservation of bumble bees. Annu Rev Entomol 53:191–208. https://doi.org/10.1146/annurev.ento.53.103106.093454
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957. https://doi.org/10.1126/science.1255957
Hartig F (2020) DHARMa: residual diagnostics for hierarchical (multi-level / mixed) regression models. R package version 0.3.1. https://CRAN.R-project.org/package=DHARMa
Heinrich B (1976) Resource partitioning among some eusocial insects: bumblebees. Ecology 57:874–889. https://doi.org/10.2307/1941054
Henry M, Rodet G (2018) Controlling the impact of the managed honeybee on wild bees in protected areas. Sci Rep 8:9308. https://doi.org/10.1038/s41598-018-27591-y
Howell AD, Alarcón R (2007) Osmia bees (Hymenoptera: Megachilidae) can detect nectar-rewarding flowers using olfactory cues. Anim Behav 74:199–205. https://doi.org/10.1016/j.anbehav.2006.11.012
Hudewenz A, Klein A-M (2015) Red mason bees cannot compete with honey bees for floral resources in a cage experiment. Ecol Evol 5:5049–5056. https://doi.org/10.1002/ece3.1762
Lenth RV (2019) emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.4.1
Lindström SAM, Herbertsson L, Rundlöf M, Bommarco R, Smith HG (2016) Experimental evidence that honeybees depress wild insect densities in a flowering crop. Proc R Soc B 283:20161641. https://doi.org/10.1098/rspb.2016.1641
Mallinger RE, Gratton C (2015) Species richness of wild bees, but not the use of managed honeybees, increases fruit set of a pollinator-dependent crop. J Appl Ecol 52:323–330. https://doi.org/10.1111/1365-2664.12377
Mallinger RE, Prasifka JR (2017) Bee visitation rates to cultivated sunflowers increase with the amount and accessibility of nectar sugars. J Appl Entomol 141:561–573. https://doi.org/10.1111/jen.12375
Mallinger RE, Gaines-Day HR, Gratton C (2017) Do managed bees have negative effects on wild bees?: a systematic review of the literature. PLoS One 12:e0189268. https://doi.org/10.1371/journal.pone.0189268
McKendrick L, Provan J, Fitzpatrick Ú, Brown MJF, Murray TE, Stolle E, Paxton RJ (2017) Microsatellite analysis supports the existence of three cryptic species within the bumble bee Bombus lucorum sensu lato. Conserv Genet 18:573–584. https://doi.org/10.1007/s10592-017-0965-3
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–260. https://doi.org/10.1016/j.biocon.2005.09.005
Müller A, Diener S, Schnyder S, Stutz K, Sedivy C, Dorn S (2006) Quantitative pollen requirements of solitary bees: implications for bee conservation and the evolution of bee–flower relationships. Biol Conserv 130:604–615. https://doi.org/10.1016/j.biocon.2006.01.023
Nielsen A, Dauber J, Kunin WE, Lamborn E, Jauker B, Moora M, Potts SG, Reitan T, Roberts S, Sõber V, Settele J, Steffan-Dewenter I, Stout JC, Tscheulin T, Vaitis M, Vivarelli D, Biesmeijer JC, Petanidou T (2012) Pollinator community responses to the spatial population structure of wild plants: a pan-European approach. Basic Appl Ecol 13:489–499. https://doi.org/10.1016/j.baae.2012.08.008
Ollerton J, Erenler H, Edwards M, Crockett R (2014) Pollinator declines. Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 346:1360–1362. https://doi.org/10.1126/science.1257259
Rose F (1981) The wildflower key: British Isles – N.W. Europe. Frederick Warne, London, United Kingdom
Rotheray EL, Osborne JL, Goulson D (2017) Quantifying the food requirements and effects of food stress on bumblebee colony development. J Apic Res 56:288–299. https://doi.org/10.1080/00218839.2017.1307712
Sáez A, Morales CL, Garibaldi LA, Aizen MA (2017) Invasive bumble bees reduce nectar availability for honey bees by robbing raspberry flower buds. Basic Appl Ecol 19:26–35. https://doi.org/10.1016/j.baae.2017.01.001
Seeley TD (1995) The wisdom of the hive: the social physiology of honeybee colonies. Harvard University Press, Cambridge
Senapathi D, Goddard MA, Kunin WE, Baldock KCR (2017) Landscape impacts on pollinator communities in temperate systems: evidence and knowledge gaps. Funct Ecol 31:26–37. https://doi.org/10.1111/1365-2435.12809
Stout JC, Morales CL (2009) Ecological impacts of invasive alien species on bees. Apidologie 40:388–409. https://doi.org/10.1051/apido/2009023
Streeter D, Hart-Davies C, Hardcastle A, Cole F, Harper L (2009) Collins flower guide: the most complete guide to the flowers of Britain and Ireland. HarperCollins Publishers, London
Taylor K (2005) Biological Flora of the British Isles: Rubus vestitus Weihe. J Ecol 93:1249–1262. https://doi.org/10.1111/j.1365-2745.2005.01076.x
Tepedino VJ, Parker FD (1982) Interspecific differences in the relative importance of pollen and nectar to bee species foraging on sunflowers. Environ Entomol 11:246–250. https://doi.org/10.1093/ee/11.1.246
Thomson DM (2016) Local bumble bee decline linked to recovery of honey bees, drought effects on floral resources. Ecol Lett 19:1247–1255. https://doi.org/10.1111/ele.12659
Torné-Noguera A, Rodrigo A, Osorio S, Bosch J (2016) Collateral effects of beekeeping: impacts on pollen-nectar resources and wild bee communities. Basic Appl Ecol 17:199–209. https://doi.org/10.1016/j.baae.2015.11.004
Villanueva-Gutiérrez R, Roubik DW, Porter-Bolland L (2015) Bee–plant interactions: competition and phenology of flowers visited by bees. In: Islebe G, Calmé S, León-Cortés J, Schmook B (eds) Biodiversity and conservation of the Yucatán Peninsula. Springer International Publishing, Switzerland
Walther-Hellwig K, Frankl R (2000) Foraging habitats and foraging distances of bumblebees, Bombus spp. (Hym., Apidae), in an agricultural landscape. J Appl Entomol 124:299–306. https://doi.org/10.1046/j.1439-0418.2000.00484.x
Walther-Hellwig K, Fokul G, Frankl R, Büchler R, Ekschmitt K, Wolters V (2006) Increased density of honeybee colonies affects foraging bumblebees. Apidologie 37:517–532. https://doi.org/10.1051/apido:2006035
Weast RC (1971) Handbook of chemistry and physics, 52nd edition (1971–1972). The Chemical Rubber Co., Cleveland
Wellington WG, Fitzpatrick SM (1981) Territoriality in the drone fly, Eristalis tenax (Diptera: Syrphidae). Can Entomol 113:695–704. https://doi.org/10.4039/Ent113695-8
Wignall VR, Campbell Harry I, Davies NL, Kenny SD, McMinn JK, Ratnieks FLW (2020a) Seasonal variation in exploitative competition between honeybees and bumblebees. Oecologia 192:351–361. https://doi.org/10.1007/s00442-019-04576-w
Wignall VR, Arscott NA, Nudds HE, Squire A, Green TO, Ratnieks FLW (2020b) Thug life: bramble (Rubus fruticosus L. agg.) is a valuable foraging resource for honeybees and diverse flower‐visiting insects. Insect Conserv Divers 13(6):543–557. https://doi.org/10.1111/icad.12436
Williams NM, Kremen C (2007) Resource distributions among habitats determine solitary bee offspring production in a mosaic landscape. Ecol Appl 17:910–921. https://doi.org/10.1890/06-0269
Williams NM, Tepedino VJ (2003) Consistent mixing of near and distant resources in foraging bouts by the solitary mason bee Osmia lignaria. Behav Ecol 14:141–149. https://doi.org/10.1093/beheco/14.1.141
Wilms W, Wiechers B (1997) Floral resource partitioning between native Melipona bees and the introduced Africanized honey bee in the Brazilian Atlantic rain forest. Apidologie 28:339–355. https://doi.org/10.1051/apido:19970602
Wojcik VA, Morandin LA, Davies Adams L, Rourke KE (2018) Floral resource competition between honey bees and wild bees: is there clear evidence and can we guide management and conservation? Environ Entomol 47:822–833. https://doi.org/10.1093/ee/nvy077
Wright IR, Roberts SPM, Collins BE (2015) Evidence of forage distance limitations for small bees (Hymenoptera: Apidae). Eur J Entomol 112:303–310. https://doi.org/10.14411/eje.2015.028
Zurbuchen A, Cheesman S, Klaiber J, Müller A, Hein S, Dorn S (2010a) Long foraging distances impose high costs on offspring production in solitary bees. J Anim Ecol 79:674–681. https://doi.org/10.1111/j.1365-2656.2010.01675.x
Zurbuchen A, Landert L, Klaiber J, Müller A, Hein S, Dorn S (2010b) Maximum foraging ranges in solitary bees: only few individuals have the capability to cover long foraging distances. Biol Conserv 143:669–676. https://doi.org/10.1016/j.biocon.2009.12.003
Acknowledgments
We particularly acknowledge Hannah Chipperfield in remembrance of her contribution to this study, and the dedication and spirit she always showed while working as part of the research team. We are also grateful to Isabella Campbell Harry, Thomas Green, Kyle Shackleton, Anna Squire and Jack Thomas for their assistance with fieldwork, and to Hayley Nudds for help with a pilot field trial. We thank the C.B. Dennis British Beekeepers’ Research Trust and the University of Sussex Doctoral School who fund VW’s PhD, of which this study is a part.
Funding
VW’s PhD, of which this study is a part, was funded by the C. B. Dennis British Beekeepers’ Research Trust and the University of Sussex Doctoral School.
Author information
Authors and Affiliations
Author notes
Hannah M. Chipperfield is deceased. This paper is dedicated to her memory.
- Hannah M. Chipperfield
Contributions
VRW and FLWR conceived the ideas and designed methodology; VRW, MB, CU, KEN and HMC collected the data; VW analysed the data; VW and FLWR led the writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
n/a
Consent to participate
n/a
Consent for publication
All authors have given final approval for publication.
Code availability
R Studio software version 1.1.463.
Additional information
Communicated by A. Toth
Dedicated to the memory of Hannah Chipperfield
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wignall, V.R., Brolly, M., Uthoff, C. et al. Exploitative competition and displacement mediated by eusocial bees: experimental evidence in a wild pollinator community. Behav Ecol Sociobiol 74, 152 (2020). https://doi.org/10.1007/s00265-020-02924-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-020-02924-y