Mucuna pruriens Administration Minimizes Neuroinflammation and Shows Anxiolytic, Antidepressant and Slimming Effects in Obese Rats
Abstract
:1. Introduction
2. Results
2.1. Chemical Characterization of MP Extract
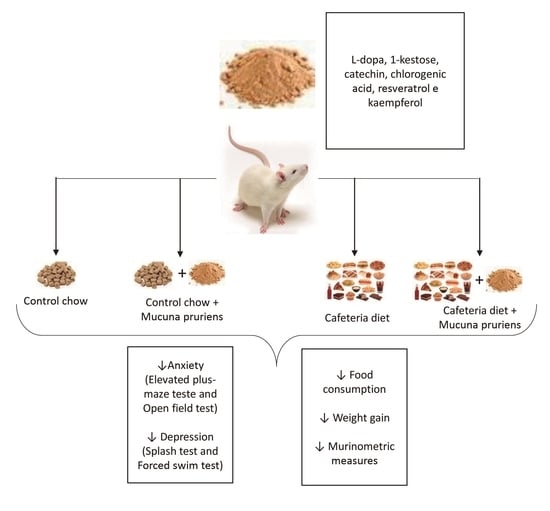
2.2. Diet Consumption, Weight Monitoring and Murinometric Parameters
2.3. Behavioral Parameters
2.4. Histological and Immunohistochemical Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and External Standards for HPLC
4.2. Preparation of the MP Extract
4.3. Chemical Composition of MP Extract
4.3.1. Proximate Composition and Total Energy Value
4.3.2. Quantification of Phenolic Profile, Organic Acids, Sugars and Oligosaccharides by High Performance Liquid Chromatography (HPLC)
4.3.3. Quantification of Levodopa by Nuclear Magnetic Resonance (1H-NMR)
4.4. Study Design
4.5. Evaluation of Behavioral Parameters
4.5.1. Anxiety-Like Behavior in Rats
4.5.2. Depression-Like Behavior in Rats
4.6. Murinometric Parameters, Euthanasia and Tissue Samples
4.7. Histological and Immunohistochemical Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- André, C.; Catania, C.; Remus-Borel, J.; Ladeveze, E.; Leste-Lasserre, T.; Mazier, W.; Binder, E.; Gonzales, D.; Clarck, S.; Guzman-Quevedo, O.; et al. mTORC1 pathway disruption abrogates the effects of the ciliary neurotrophic factor on energy balance and hypothalamic neuroinflammation. Brain Behav. Immun. 2018, 70, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Lakkis, J.I.; Weir, M.R. Obesity and Kidney Disease. Prog. Cardiovasc. Dis. 2018, 61, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajan, T.M.; Menon, V. Psychiatric disorders and obesity: A review of association studies. J. Postgrad. Med. 2017, 63, 182–190. [Google Scholar] [PubMed]
- Delgado, I.; Huet, L.; Dexpert, S.; Beau, C.; Forestier, D.; Ledaguenel, P.; Aubert, A.; Sauvant, J.; Aouizerate, B.; Magne, E.; et al. Depressive symptoms in obesity: Relative contribution of low-grade inflammation and metabolic health. Psychoneuroendocrinology 2018, 91, 55–61. [Google Scholar] [CrossRef]
- Guillemot-Legris, O.; Muccioli, G.G. Obesity-Induced Neuroinflammation: Beyond the Hypothalamus. Trends Neurosci. 2017, 40, 237–253. [Google Scholar] [CrossRef]
- Liu, W.; Ge, T.; Leng, Y.; Pan, Z.; Fan, J.; Yang, W.; Cui, R. The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast. 2017, 2017, 6871089. [Google Scholar] [CrossRef] [Green Version]
- Mah, L.; Szabuniewicz, C.; Fiocco, A.J. Can anxiety damage the brain? Curr. Opin. Psychiatry 2016, 29, 56–63. [Google Scholar] [CrossRef]
- Erta, M.; Giralt, M.; Esposito, F.L.; Fernandez-Gayol, O.; Hidalgo, J. Astrocytic IL-6 mediates locomotor activity, exploration, anxiety, learning and social behavior. Horm. Behav. 2015, 73, 64–74. [Google Scholar] [CrossRef]
- Renata, L.T.; Alexandre, S.S.; Ana, R.N.C.; Alexandre, R.P.S.; Jailane, S.A. Nutritional composition, phytochemicals and microbiological quality of the legume, Mucuna pruriens. Afr. J. Biotechnol. 2015, 14, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Bala, V.; Debnath, A.; Shill, A.K.; Bose, U. Anti-Inflammatory, Diuretic and Antibacterial Activities of Aerial Parts of Mucuna pruriens Linn. Int. J. Pharm. 2011, 7, 498–503. [Google Scholar] [CrossRef]
- Tripathi, Y.B.; Upadhyay, A.K. Effect of the alcohol extract of the seeds of Mucuna pruriens on free radicals and oxidative stress in albino rats. Phytother. Res. 2002, 16, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Pai, P.G.; Rajeshwari, S.; Ullal, S.D.; Nishith, R.S.; Belagali, Y. Evaluation of Anxiolytic Effect of Chronic Administration of Mucuna pruriens in Wistar Albino Rats. Am. J. Pharm. Tech. Res. 2014, 4, 611–619. [Google Scholar]
- Sachan, A.; Kuma, S.; Singh, H.; Shankar, P.; Kumar, D.; Sachan, A.K.; Dixit, R.K. Potential Anti-Anxiety Effect of Mucuna pruriens in Experimental Model of Swiss Albino Mice. PTB Rep. 2015, 1, 20–23. [Google Scholar] [CrossRef] [Green Version]
- Nicoletti, C.F.; Delfino, H.B.P.; Ferreira, F.C.; Pinhel, M.A.S.; Nonino, C.B. Role of eating disorders-related polymorphisms in obesity pathophysiology. Rev. Endocr. Metab. Disord. 2019, 20, 115–125. [Google Scholar] [CrossRef]
- Agbafor, K.N.; Nwachukwu, N. Phytochemical Analysis and Antioxidant Property of Leaf Extracts of Vitex doniana and Mucuna pruriens. Biochem. Res. Int. 2011, 2011, 459839. [Google Scholar] [CrossRef] [Green Version]
- Gulati, V.; Harding, I.H.; Palombo, E.A. Enzyme inhibitory and antioxidant activities of traditional medicinal plants: Potential application in the management of hyperglycemia. BMC Complement. Altern. Med. 2012, 12, 77. [Google Scholar] [CrossRef] [Green Version]
- Handajani, S. Indigenous mucuna tempe as functional food. Asia Pac. J. Clin. Nutr. 2001, 10, 222–225. [Google Scholar] [CrossRef]
- Vadivel, V.; Janardhanan, K. Nutritional and antinutritional characteristics of seven South Indian wild legumes. Plant. Foods Hum. Nutr. 2005, 60, 69–75. [Google Scholar] [CrossRef]
- Agustí, A.; Garcia-Pardo, M.P.; López-Almela, I.; Campillo, I.; Maes, M.; Romani-Pérez, M.; Sanz, Y. Interplay between the Gut-Brain Axis, Obseity and Cognitive Function. Front. Neurosci. 2018, 12, 155. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef] [Green Version]
- Tochio, T.; Kadota, Y.; Tanaka, T.; Koga, Y. 1-Kestose, the Smallest Fructooligosaccharide Component, Which Efficiently Stimulates Faecalibacterium prausnitzii as Well as Bifidobacteria in Humans. Foods 2018, 7, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Physiol. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.R.; Singh, S.; Youssef, F.F. Cafeteria-diet induced obesity results in impaired cognitive functioning in a rodent model. Heliyon 2019, 5, e01412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neeland, I.J.; Ross, R.; Després, J.-P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Cavalcanti, C.L.; Gonçalves, M.C.R.; Alves, A.F.; de Araújo, E.V.; Carvalho, J.L.P.; Lins, P.P.; Alves, R.C.; Soares, N.L.; Pordeus, L.C.M.; Aquino, J.S. Antidepressant, Anxiolytic and Neuroprotective Activities of Two Zinc Compounds in Diabetic Rats. Front. Neurosci. 2019, 13, 1411. [Google Scholar] [CrossRef]
- Estrela, D.C.; da Silva, W.A.M.; Guimarães, A.T.B.; Mendes, B.O.; Castro, A.L.; Torres, I.L.S.; Malafaia, G. Predictive behaviors for anxiety and depression in female Wistar rats subjected to cafeteria diet and stress. Physiol. Behav. 2015, 151, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Liu, J.; Gao, J.; Wu, X.; Cui, C.; Wei, H.; Zheng, R.; Peng, J. Combined Soluble Fiber-Mediated Intestinal Microbiota Improve Insulin Sensitivity of Obese Mice. Nutrients 2020, 12, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kita, M.; Uchida, S.; Yamada, K.; Ano, Y. Anxiolytic effects of theaflavins via dopaminergic activation in the frontal cortex. Biosci. Biotechnol. Biochem. 2019, 83, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Meza, S.; Rodríguez-Landa, J.F.; Martínez, A.J.; Herrera-Meza, G.; Fernández-Demeneghi, R.; Reyes-Saldaña, K.; Oliart-Ros, R.M. Behavioral Effect of Sterculia apetala Seed Oil Consumption in Male Zucker Rats. J. Med. Food. 2017, 20, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Shieh, K.-R.; Yang, S.-C. Formosan wood mice (Apodemus semotus) exhibit more exploratory behaviors and central dopaminergic activities than C57BL/6 mice in the open field test. Chin. J. Physiol. 2020, 63, 27–34. [Google Scholar] [PubMed]
- Selyatitskaya, V.G.; Kuzminova, O.I. Emotional and behavioral reactions in experimental animals with alimentary obesity. Bull. Exp. Biol. Med. 2008, 146, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Lee, G.; Chen, J.-H.; Huizinga, J.D. Relationships between Distention-, Butyrate- and Pellet-Induced Stimulation of Peristalsis in the Mouse Colon. Front. Physiol. 2020, 11, 109. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Gupta, P.S.; Gupta, R. Evaluation of anti-anxiety activity of Mucuna pruriens. J. Drug Deliv. 2019, 9, 104–107. [Google Scholar] [CrossRef]
- Pati, D.; Pandey, D.K.; Mahesh, R.; Jhavad, V.K.H.R. Anti-Depressant-Like Activity of Mucuna pruriens; A Traditional Indian Herb in Rodent Models of Depression. Phamacology Online 2010, 1, 537–551. [Google Scholar]
- Pulikkalpura, H.; Kurup, R.; Mathew, P.J.; Baby, S. Levodopa in Mucuna pruriens and its degradation. Sci. Rep. 2015, 5, 11078. [Google Scholar] [CrossRef]
- Moore, A.; Beidler, J.; Hong, M.Y. Resveratrol and Depression in Animal Models: A Systematic Review of the Biological Mechanisms. Molecules 2018, 23, 2197. [Google Scholar] [CrossRef] [Green Version]
- Siddhuraju, P.; Becker, K. Nutritional and antinutritional composition, in vitro amino acid availability, starch digestibility and predicted glycemic index of differentially processed mucuna beans (Mucuna pruriens var. utilis): An under-utilised legume. Food Chem. 2005, 91, 275–286. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Vijayakumari, K.; Janardhanan, K. Chemical Composition and Protein Quality of the Little-Known Legume, Velvet Bean (Mucuna pruriens (L.) DC.). J. Agric. Food Chem. 1996, 44, 2636–2641. [Google Scholar] [CrossRef]
- Hu, C.; Luo, Y.; Wang, H.; Kuang, S.; Liang, G.; Yang, Y.; Mai, S.; Yang, J. Re-evaluation of the interrelationships among the behavioral tests in rats exposed to chronic unpredictable mild stress. PLoS ONE 2017, 12, e0185129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boldarine, V.T.; Pedroso, A.P.; Neto, N.I.P.; Dornellas, A.P.S.; Nascimento, C.M.O.; Oyama, L.M.; Ribeiro, E.B. High-fat diet intake induces depressive-like behavior in ovariectomized rats. Sci. Rep. 2019, 9, 10551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matarazzo, I.; Toniato, E.; Robuffo, I. Psychobiome Feeding Mind: Polyphenolics in Depression and Anxiety. Curr. Top. Med. Chem. 2018, 18, 2108–2115. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 2015, 97, 52587. [Google Scholar] [CrossRef]
- Rana, D.G.; Galani, V.J. Dopamine mediated antidepressant effect of Mucuna pruriens seeds in various experimental models of depression. AYU 2014, 35, 90–97. [Google Scholar]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef]
- Şahin, T.D.; Göçmez, S.S.; Eraldemir, F.C.; Utkan, T. Anxiolytic-Like and Antidepressant-Like Effects of Resveratrol in Streptozotocin-Induced Diabetic Rats. Arch. Neuropsychiatry 2019, 56, 144–149. [Google Scholar]
- Souza, D.O.; Sales, V.S.; Rodrigues, C.K.S.; Oliveira, L.R.; Lemos, S.I.C.; Delmondes, G.A.; Monteiro, A.B.; Nascimento, E.P.; Figueirêdo, F.R.S.D.N.; Costa, J.G.M.; et al. Phytochemical Analysis and Central Effects of Linnaeus: Possible Involvement of the Gabaergic and Monoaminergic Systems. Iran. J. Pharm. Res. 2018, 17, 1306–1317. [Google Scholar]
- Wang, S.-N.; Ding, Y.-S.; Ma, X.-J.; Zhao, C.-B.; Lin, M.-X.; Luo, J.; Jiang, Y.-N.; He, S.; Guo, J.-Y.; Shi, J.-L. Identification of Bioactive Chemical Markers in Improving Anxiety in Rat by Fingerprint-Efficacy Study. Molecules 2018, 23, 2329. [Google Scholar] [CrossRef] [Green Version]
- Haissaguerre, M.; Ferrière, A.; Simon, V.; Saucisse, N.; Dupuy, N.; André, C.; Clark, S.; Guzman-Quevedo, O.; Tabarin, A.; Cota, D. mTORC1-dependent increase in oxidative metabolism in POMC neurons regulates food intake and action of leptin. Mol. Metab. 2018, 12, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, S.; Ota, K.T.; Wohleb, E.S.; Rasmussen, K.; Duman, R.S. High-Fat Diet Induced Anxiety and Anhedonia: Impact on Brain Homeostasis and Inflammation. Neuropsychopharmacology 2016, 41, 1874–1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, A.P.; Soares, A.L.G.; Menezes, A.M.B.; Assunção, M.C.; Wehrmeister, F.C.; Howe, L.D.; Gonçalves, H. Adiposity, depression and anxiety: Interrelationship and possible mediators. Rev. Saude Publica 2019, 53, 103. [Google Scholar] [CrossRef] [PubMed]
- Muthu, K.; Krishnamoorthy, P. Evaluation of androgenic activity of Mucuna pruriens in male rats. Afr. J. Biotechnol. 2011, 10, 15017–15019. [Google Scholar] [CrossRef] [Green Version]
- Association of Official Agricultural Chemistry. Official Methods of Analysis; Association of Official Agricultural Chemistry: Gaithersburg, Maryland, 2019. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Prosky, L.; Asp, N.G.; Schweizer, T.F.; DeVries, J.W.; Furda, I.; Lee, S.C. Determination of soluble dietary fiber in foods and food products: Collaborative study. J. AOAC Int. 1994, 77, 690–694. [Google Scholar] [CrossRef]
- Lima, M.S.; Nunes, P.C.; Silva, B.L.A.; Padilha, C.V.S.; Bonfim, T.H.F.; Stamford, T.L.M.; Vasconcelos, M.A.S.; Aquino, J.S. Determining 1-kestose, nystose and raffinose oligosaccharides in grape juices and wines using HPLC: Method validation and characterization of products from Northeast Brazil. J. Food Sci. Technol. 2019, 56, 4575–4584. [Google Scholar] [CrossRef]
- Coelho, E.M.; Padilha, C.V.S.; Miskinis, G.A.; Sá, A.G.B.; Pereira, G.E.; Azevêdo, L.C.; Lima, M.S. Simultaneous analysis of sugars and organic acids in wine and grape juices by HPLC: Method validation and characterization of products from northeast Brazil. J. Food Compost Anal. 2018, 66, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Padilha, C.V.S.; Miskinis, G.A.; Souza, M.E.A.O.; Pereira, G.E.; Oliveira, D.; Bordignon-Luiz, M.T.; Lima, M.S. Rapid determination of flavonoids and phenolic acids in grape juices and wines by RP-HPLC/DAD: Method validation and characterization of commercial products of the new Brazilian varieties of grape. Food Chem. 2017, 228, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Dutra, M.C.P.; Rodrigues, L.L.; Oliveira, D.; Pereira, G.E.; Lima, M.D.S. Integrated analyses of phenolic compounds and minerals of Brazilian organic and conventional grape juices and wines: Validation of a method for determination of Cu, Fe and Mn. Food Chem. 2018, 269, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals; Institute for Laboratory Animal Research; Division on Earth and Life Studies; National Research Council. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Leffa, D.D.; Santos, C.E.I.; Daumann, F.; Longaretti, L.M.; Amaral, L.; Dias, J.F.; Silva, J.; Andrade, V.M. Effects of Supplemental Acerola Juice on the Mineral Concentrations in Liver and Kidney Tissue Samples of Mice Fed with Cafeteria Diet. Biol. Trace Elem. Res. 2015, 167, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Bonfim, T.H.F.; Tavares, R.L.; Vasconcelos, M.H.; Gouveia, M.; Nunes, P.C.; Soares, N.L.; Alves, R.C.; Carvalho, J.L.P.; Alves, A.F.; Pereira, R.A.; et al. Potentially obesogenic diets alter metabolic and neuro-behavioural parameters in Wistar rats: A comparison between two dietary models. J. Affec Disord. 2020, 279, 451–461. [Google Scholar]
- Manalisha, D.; Chandra, K.J. Preliminary phytochemical analysis and acute oral toxicity study of Mucuna pruriens Linn. in albino mice. Int. Res. J. Pharm. 2012, 3, 181–183. [Google Scholar]
- Vadivel, V.; Pugalenthi, M. Studies on the incorporation of velvet bean (Mucuna pruriens var. utilis) as an alternative protein source in poultry feed and its effect on growth performance of broiler chickens. Trop Anim. Health Prod. 2010, 42, 1367–1376. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Grundmann, O.; Nakajima, J.-I.; Seo, S.; Butterweck, V. Anti-anxiety effects of Apocynum venetum L. in the elevated plus maze test. J. Ethnopharmacol. 2007, 110, 406–411. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharm. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Walsh, R.N.; Cummins, R.A. The Open-Field Test: A critical review. Psychol. Bull. 1976, 83, 482–504. [Google Scholar] [CrossRef]
- Filho, C.B.; Jesse, C.R.; Donato, F.; Del Fabbro, L.; Gomes, M.G.; Goes, A.T.R.; Souza, L.C.; Giacomeli, R.; Antunes, M.; Luchese, C.; et al. Neurochemical factors associated with the antidepressant-like effect of flavonoid chrysin in chronically stressed mice. Eur. J. Pharmacol. 2016, 791, 284–296. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Yamamoto, M.; Tagawa, N.; Kobayashi, Y.; Mitsui-Saitoh, K.; Hotta, Y.; Yamada, J. Differences between mice strains in response to paroxetine in the forced swimming test: Involvement of serotonergic or noradrenergic systems. Eur. J. Pharm. 2011, 672, 121–125. [Google Scholar] [CrossRef]
- Novelli, E.L.B.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.H.; Cicogna, A.C.; Novelli Filho, J.L.V.B. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardis, L.L.; Patterson, B.D. Correlation between “Lee index” and carcass fat content in weanling and adult female rats with hypothalamic lesions. J. Endocrinol. 1968, 40, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Yakaiah, V.; Dakshinamoorthi, A.; Kavimani, S. Effect of Myristica fragrans extract on total body composition in cafeteria diet induced obese rats. Bioinformation 2019, 15, 657–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prata, L.O.; Rodrigues, C.R.; Martins, J.M.; Vasconcelos, P.C.; Oliveira, F.M.S.; Ferreira, A.J.; Rodrigues-Macchado, M.G.; Caliari, M.V. Original Research: ACE2 activator associated with physical exercise potentiates the reduction of pulmonary fibrosis. Exp. Biol. Med. 2017, 242, 8–21. [Google Scholar] [CrossRef] [Green Version]
- Aniszewska, A.; Chłodzińska, N.; Bartkowska, K.; Winnicka, M.M.; Turlejski, K.; Djavadian, R.L. The expression of interleukin-6 and its receptor in various brain regions and their roles in exploratory behavior and stress responses. J. Neuroimmunol. 2015, 284, 1–9. [Google Scholar] [CrossRef]







| Parameters | Mean and Standard Deviation |
|---|---|
| Moisture (g/100 g) | 3.34 ± 0.60 |
| Ash (g/100 g) | 2.63 ± 0.26 |
| Protein (g/100 g) | 7.11 ± 0.30 |
| Lipid (g/100 g) | 0.41 ± 0.05 |
| Carbohydrate (g/100 g) | 86.63 ± 0.27 |
| Monosaccharide and disaccharide (mg/g) | |
| Fructose | 12.65 ± 0.03 |
| Glucose | 17.62 ± 0.05 |
| Maltose | 7.74 ± 0.02 |
| Dietary fiber (g/100 g) | |
| IDF | 1.01 ± 0.54 |
| SDF | 1.42 ± 0.02 |
| TDF | 2.43 |
| IDF:SDF ratio | 0.71 |
| Total energy value (kJ/100 g) | 1579.86 ± 7.22 |
| Total energy value (kcal/100 g) | 377.59 ± 1.73 |
| Oligosaccharide (mg/100 g) | |
| 1-Kestose | 20.70 |
| Nystose | n.d. |
| Raffinose | n.d. |
| Organic acids (mg/100 g) | |
| Citric | 31.76 ± 0.06 |
| Tartaric | 8.57 ± 0.04 |
| Formic | 18.15 ± 0.08 |
| Levodopa (%) | 14.08 ± 0.08 |
| Phenolic compounds (mg/100 g) | |
| Flavanol | |
| Catechin | 57.83 ± 0.06 |
| Procyanidin B1 | 16.44 ± 0.09 |
| Procyanidin B2 | 18.41 ± 0.08 |
| Flavonol | |
| Quercitin 3-Glucoside | 13.00 ± 0.05 |
| Kaempferol 3-Glucoside | 19.34 ± 0.04 |
| Total flavonoids | 125.02 |
| Phenolic acids | |
| Chlorogenic acid | 49.32 ± 0.06 |
| Stilbenes | |
| Trans-resveratrol | 21.45 ± 0.05 |
| Total non-flavonoids | 70.77 |
| Total phenolic compounds | 195.79 |
| Parameters | Groups | |||
|---|---|---|---|---|
| HG | HGMP | OG | OGMP | |
| Body length (cm) | 23.2 ± 0.91 | 22.63 ± 0.64 # γ | 24.75 ± 0.88 * | 24.0 ± 0.55 |
| Abdominal circumference (cm) | 15.33 ± 0.82 | 15.78 ± 0.67 # | 19.08 ± 1.24 * | 16.5 ± 0.79 # |
| Thoracic circumference (cm) | 14.67 ± 0.68 | 14.75 ± 0.6 # γ | 16.92 ± 0.2 * | 15.93 ± 0.53 # * |
| BMI (g/cm2) | 0.71 ± 0.04 | 0.71 ± 0.03 # | 0.80 ± 0.04 * | 0.73 ± 0.03 # |
| Lee Index | 0.31 ± 0.01 | 0.32 ± 0.01 | 0.32 ± 0.01 | 0.32 ± 0.01 |
Sample Availability: Samples of the compounds are available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, R.l.; Vasconcelos, M.H.A.d.; Dutra, M.L.d.V.; D’Oliveira, A.B.; Lima, M.d.S.; Salvadori, M.G.d.S.S.; Pereira, R.d.A.; Alves, A.F.; Nascimento, Y.M.d.; Tavares, J.F.; et al. Mucuna pruriens Administration Minimizes Neuroinflammation and Shows Anxiolytic, Antidepressant and Slimming Effects in Obese Rats. Molecules 2020, 25, 5559. https://doi.org/10.3390/molecules25235559
Tavares Rl, Vasconcelos MHAd, Dutra MLdV, D’Oliveira AB, Lima MdS, Salvadori MGdSS, Pereira RdA, Alves AF, Nascimento YMd, Tavares JF, et al. Mucuna pruriens Administration Minimizes Neuroinflammation and Shows Anxiolytic, Antidepressant and Slimming Effects in Obese Rats. Molecules. 2020; 25(23):5559. https://doi.org/10.3390/molecules25235559
Chicago/Turabian StyleTavares, Renata leite, Maria Helena Araújo de Vasconcelos, Maria Letícia da Veiga Dutra, Aline Barbosa D’Oliveira, Marcos dos Santos Lima, Mirian Graciela da Silva Stiebbe Salvadori, Ramon de Alencar Pereira, Adriano Francisco Alves, Yuri Mangueira do Nascimento, Josean Fechine Tavares, and et al. 2020. "Mucuna pruriens Administration Minimizes Neuroinflammation and Shows Anxiolytic, Antidepressant and Slimming Effects in Obese Rats" Molecules 25, no. 23: 5559. https://doi.org/10.3390/molecules25235559