On the Recovery of Hematite from an Iron Ore Fine Fraction by Electroflotation Using a Biosurfactant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mineral Samples
2.2. Preparing and Obtaining the Biosurfactant
2.3. Surface Properties of the Mineral
2.4. Electroflotation Test
3. Results
3.1. Iron Ore Characterization
3.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.3. Surface Tension Studies
3.4. Zeta Potential Studies
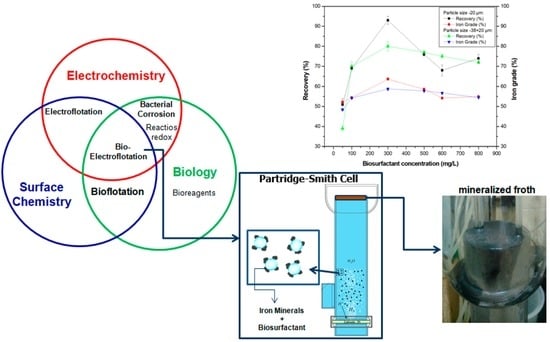
3.5. Electroflotation Test
3.5.1. Effect of pH
3.5.2. Effect of the Biosurfactant Concentration
3.5.3. Effect of Current Density
3.5.4. Influence of System Agitation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prakash, S.; Das, B.; Mohanty, J.; Venugopal, R. The recovery of fine iron minerals from quartz and corundum mixtures using selective magnetic coating. Int. J. Miner. Process. 1999, 57, 87–103. [Google Scholar] [CrossRef]
- Espósito, T.J.; Duarte, A.P. Risk-factor classification of tailings and industrial waste dams. Rem Rev. Esc. Minas 2010, 63, 393–398. [Google Scholar] [CrossRef]
- Rubio, J.; Capponi, F.; Matiolo, E.; Nune, S.D. Advances in the flotation of copper and molybdenum sulphide ore fines. In Proceedings of the XXI Brazilian Meeting on Mineral Processing and Extractive Metallurgy, Natal, Rio Grande do Norte, Brazil, 15 November 2005; pp. 69–78. [Google Scholar]
- Waters, K.E.; Hadler, K.; Cilliers, J. The flotation of fine particles using charged microbubbles. Miner. Eng. 2008, 21, 918–923. [Google Scholar] [CrossRef]
- Shahbazi, B.; Rezai, B.; Koleini, S.M.J. Bubble–particle collision and attachment probability on fine particles flotation. Chem. Eng. Process. Process. Intensif. 2010, 49, 622–627. [Google Scholar] [CrossRef]
- Pease, J.; Curry, D.; Young, M. Designing flotation circuits for high fines recovery. Miner. Eng. 2006, 19, 831–840. [Google Scholar] [CrossRef]
- Miettinen, T.; Ralston, J.; Fornasiero, D. The limits of fine particle flotation. Miner. Eng. 2010, 23, 420–437. [Google Scholar] [CrossRef]
- Yao, J.; Yin, W.; Gong, E. Depressing effect of fine hydrophilic particles on magnesite reverse flotation. Int. J. Miner. Process. 2016, 149, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Solongo, S.K.; Gomez-Flores, A.; You, J.; Choi, S.; Heyes, G.W.; Ilyas, S.; Lee, J.; Kim, H. Cationic collector conformations on an oxide mineral interface: Roles of pH, ionic strength, and ion valence. Miner. Eng. 2020, 150, 106277. [Google Scholar] [CrossRef]
- Alam Sarkar, S.K.; Evans, G.M.; Donne, S.W. Bubble size measurement in electroflotation. Miner. Eng. 2010, 23, 1058–1065. [Google Scholar] [CrossRef]
- Hacha, R.R.; Torem, M.L.; Merma, A.G.; Coelho, V.F.D.S. Electroflotation of fine hematite particles with Rhodococcus opacus as a biocollector in a modified Partridge–Smith cell. Miner. Eng. 2018, 126, 105–115. [Google Scholar] [CrossRef]
- Bagotsky, V.S. Industrial Electrolytic Processes. Fundam. Electrochem. 2005, 319–325. [Google Scholar] [CrossRef]
- Ketkar, D.; Mallikarjunan, R.; Venkatachalam, S. Electroflotation of quartz fines. Int. J. Miner. Process. 1991, 31, 127–138. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Gomez-Flores, A.; Solongo, S.K.; Heyes, G.W.; Ilyas, S.; Kim, H. Bubble−particle interactions with hydrodynamics, XDLVO theory, and surface roughness for flotation in an agitated tank using CFD simulations. Miner. Eng. 2020, 152, 106368. [Google Scholar] [CrossRef]
- Bezerra, C.G. Characterization of iron ore tailings (IOT) and evaluation of its influence on the physical-chemical and mechanical behavior of cement pastes. Master’s Thesis, (Specialization in Civil Engineering) Federal University of Rio de Janeiro, Rio de Janeiro, Brazil, 2017. (In Portuguese). [Google Scholar]
- Mesquita, L.M.S.; Torem, M.L.; Lins, F.F. Interaction of a hydrophonic bacterium strain in a hematite-quartz flotation system. Int. J. Miner. Process. 2003, 71, 31–44. [Google Scholar] [CrossRef]
- Simões, C.R. Electroflotation of fine and ultra-fine iron ore with the use of a biosurfactant extracted from Rhodococcus opacus bacteria. Master’s Thesis, (Specialization in Chemical and Materials Engineering) Pontifical Catholic University of Rio de Janeiro, Rio de Janeiro, Brazil, 2020. (In Portuguese). [Google Scholar]
- Huang, X.; Xiao, W.; Zhao, H.; Cao, P.; Hu, Q.; Qin, W.; Zhang, Y.; Qiu, G.; Wang, J. Hydrophobic flocculation flotation of rutile fines in presence of styryl phosphonic acid. Trans. Nonferr. Met. Soc. China 2018, 28, 1424–1432. [Google Scholar] [CrossRef]
- Maity, D.; Agrawal, D. Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous media. J. Magn. Magn. Mater. 2007, 308, 46–55. [Google Scholar] [CrossRef]
- Neto, F.N.S. Preparation and characterization of magnetic iron oxides coated with polydimethylsiloxane. Master’s Thesis, (Specialization in Materials Engineering) State University of Goiás, Anapolis, Brazil, 2012. (In Portuguese). [Google Scholar]
- Ashkenazy, R.; Gottlieb, L.; Yannai, S. Characterization of acetone-washed yeast biomass functional groups involved in lead biosorption. Biotechnol. Bioeng. 1997, 55, 1–10. [Google Scholar] [CrossRef]
- Kapoor, A.; Viraraghavan, T. Heavy metal biosorption sites in Aspergillus niger. Bioresour. Technol. 1997, 61, 221–227. [Google Scholar] [CrossRef]
- Merma, A.G.; Torem, M.L.; Morán, J.J.; Monte, M.B.M. On the fundamental aspects of apatite and quartz flotation using a Gram positive strain as a bioreagent. Miner. Eng. 2013, 48, 61–67. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Ju, M.; Li, X.; Yu, Q. Purification and characterization of biosurfactant produced by Bacillus licheniformis Y-1 and its application in remediation of petroleum contaminated soil. Mar. Pollut. Bull. 2016, 107, 46–51. [Google Scholar] [CrossRef]
- Olivera, C.A.C. Flotation of the hematite-quartz system using the soluble biosurfactant produced by Rhodococcus erythropolis. Ph.D. Thesis, (Specialization in Chemical and Materials Engineering) Pontifical Catholic University of Rio de Janeiro, Rio de Janeiro, Brazil, 2018. (In Portuguese). [Google Scholar]
- Deo, N.; Natarajan, K.; Somasundaran, P. Mechanisms of adhesion of Paenibacillus polymyxa onto hematite, corundum and quartz. Int. J. Miner. Process. 2001, 62, 27–39. [Google Scholar] [CrossRef]
- Szymańska, A.; Sadowski, Z. Effects of biosurfactants on surface properties of hematite. Adsorption 2010, 16, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Makri, E.A.; Doxastakis, G.I. Surface tension of Phaseolus vulgaris and coccineus proteins and effect of pollysaccharides on their foaming properties. Food Chem. 2007, 101, 37–48. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Ivshina, I.B. Rhodococcus Biosurfactants: Biosynthesis, Properties, and Potential Applications. In Biology of Rhodococcus; Alvarez, H.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 291–313. [Google Scholar]
- Mulligan, C.; Sharma, S.; Mudhoo, A. Biosurfactants Research Trends and Applications; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Lang, S.; Philp, J.C. Surface-active lipids in Rhodoccocus. J. Microbiol. Antonie Van Leeuwenhoek 1998, 74, 59–70. [Google Scholar] [CrossRef]
- Olivera, C.A.C.; Merma, A.G.; Puelles, J.G.S.; Torem, M.L. On the fundamentals aspects of hematite bioflotation using a Gram positive strain. Miner. Eng. 2017, 106, 55–63. [Google Scholar] [CrossRef]
- Camarate, M.C. Selective bioflocculation of ultra-fine hematite contained in iron ore tailings using Candido stellata yeast. Master’s Dissertation, (Department of Chemical Engineering and Materials), Pontifical Catholic University of Rio de Janeiro, Rio de Janeiro, Brazil, 2019. (In Portuguese). [Google Scholar]
- Kim, G.; Park, K.; Choi, J.; Gomez-Flores, A.; Han, Y.; Choi, S.Q.; Kim, H. Bioflotation of malachite using different growth phases of Rhodococcus opacus: Effect of bacterial shape on detachment by shear flow. Int. J. Miner. Process. 2015, 143, 98–104. [Google Scholar] [CrossRef]
- Ekmekyapar, F.; Aslan, A.; Bayhan, K.Y.; Cakici, A. Biosorption of copper (II) by nonliving lichen biomass of Cladonia rangiformis hoffm. J. Hazard. Mater. 2006, 137, 293–298. [Google Scholar] [CrossRef]
- Jiménez, C.; Talavera, B.; Sáez, C.; Canizares, P.; Rodrigo, M. Study of the production of hydrogen bubbles at low current densities for electroflotation processes. J. Chem. Technol. Biotechnol. 2010, 85, 1368–1373. [Google Scholar] [CrossRef]
- Yoon, R.-H. The role of hydrodynamic and surface forces in bubble–particle interaction. Int. J. Miner. Process. 2000, 58, 129–143. [Google Scholar] [CrossRef]
- Modirshahla, N.; Behnajady, M.; Mohammadi-Aghdam, S. Investigation of the effect of different electrodes and their connections on the removal efficiency of 4-nitrophenol from aqueous solution by electrocoagulation. J. Hazard. Mater. 2008, 154, 778–786. [Google Scholar] [CrossRef] [PubMed]












| Sample | Composition | ||
|---|---|---|---|
| Fe (%) | Fe2O3 (%) | SiO2 (%) | |
| A: −38 + 20 µm | 53.84 ± 3 | 76.91 | 23.09 |
| B: −20 µm | 54.06 ± 3 | 77.22 | 22.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simões, C.R.; Hacha, R.R.; Merma, A.G.; Torem, M.L. On the Recovery of Hematite from an Iron Ore Fine Fraction by Electroflotation Using a Biosurfactant. Minerals 2020, 10, 1057. https://doi.org/10.3390/min10121057
Simões CR, Hacha RR, Merma AG, Torem ML. On the Recovery of Hematite from an Iron Ore Fine Fraction by Electroflotation Using a Biosurfactant. Minerals. 2020; 10(12):1057. https://doi.org/10.3390/min10121057
Chicago/Turabian StyleSimões, Carolina R., Ronald R. Hacha, Antonio G. Merma, and Maurício L. Torem. 2020. "On the Recovery of Hematite from an Iron Ore Fine Fraction by Electroflotation Using a Biosurfactant" Minerals 10, no. 12: 1057. https://doi.org/10.3390/min10121057