Abstract
Interspecific F1 hybrids were obtained from crosses between two Ogura cytoplasmic male sterility (Ogura CMS) broccoli lines (B14 and 137) and a rapeseed restorer line to introgress the Ogura CMS’s fertility-restoration gene Rfo from rapeseed into broccoli. Ten Rfo-positive interspecific, triploid plant progenies, phenotypically between rapeseed and broccoli, were obtained but still contained multiple rapeseed genes, with reduced fertility and seed setting potential under natural pollination. For fertility and seed setting improvement, successive backcrosses with broccoli were carried out to yield BC2 progenies. Screening revealed six plants with Rfo among 25 BC2 progenies, which were investigated for agronomic traits, ploidy determination, pollen viability and SSR (simple sequence repeat) markers to evaluate for ideal individuals closer to broccoli. Pollen viability tests were carried out at different stages for various hybrid parents. The results were as follows: 137-F1 (68.54%) > B14-F1 (65.32%) > 137-BC1 (48.15%) > B14-BC1 (32.41%). Although there were significant differences among the BC1 and BC2 individuals, the genetic background of BC2 was closer to the parent broccoli compared with that of BC1 plants. The existence of the Rfo gene in broccoli not only facilitates broccoli germplasm innovation, combination of interspecific hybridization and backcrossing, but may also shed light on trait segregation in different generations.




Similar content being viewed by others
References
Ayotte R, Harney P, Machado VS (1987) The transfer of triazine resistance from Brassica napus L. to B. oleracea LI Production of F 1 hybrids through embryo rescue. Euphytica 36:615–624
Chamola R, Balyan H, Bhat S (2013) Transfer of cytoplasmic male sterility from alloplasmic Brassica juncea and B. napus to cauliflower (B. oleracea var. botrytis) through interspecific hybridization and embryo culture. Ind J Genet 73:203–210
Chen Y, Wojciechowski A (2000) Obtained interspecific hybrid of B. napus and B. oleracea var. italica by embryo and ovule in vitro culture. J Huazhong (Cent China) Agric Univ 19:274–278
Chen C, Zhuang M, Fang ZY, Wang QB, Zhang YY, Liu YM, Yang LM, Cheng F (2013a) A co-dominant marker BoE332 applied to marker-assisted selection of homozygous male-sterile plants in cabbage (Brassica oleracea var. capitata L.). Journal of Integrative Agriculture 12:596–602
Chen W, Li M, Wang T, Hui R, Tu J, Fu T (2013b) Development of new restorer materials with Ogu CMS in Brassica napus. Agric Sci Technol 14:18
Chen J, Luo M, Li SN, Tao M, Ye X, Duan W, Zhang C, Qin Q, Xiao J, Liu SJ (2018) A comparative study of distant hybridization in plants and animals science. China Life Sci 61:285–309
Delourme R, Budar F (1999) Male sterility. In: Gómez-Campo C (ed) Biology of Brassica coenospecies. Elsevier, Amsterdam, pp 185–216
Dixon GR (2007) Vegetable brassicas and related crucifers, vol 14. CABI, Wallingford
Doležel J, Bartoš J (2005) Plant DNA flow cytometry and estimation of nuclear genome size. Ann Bot 95:99–110
Fang Z, Sun P, Liu Y, Yang L, Wang X, Hou A, Bian C (1979) A male sterile line with dominant gene (Ms) in cabbage (Brassica oleracea var. capitata) and its utilization for hybrid seed production. Euphytica 97:265–268
Fang Z, Liu Y, Yang L, Wang X, Zhuang M, Zhang Y, Sun P (2004) Breeding and seed production technique of dominant genic male sterile (DGMS) line and cytoplasmic male sterile (CMS) line in cabbage. Sci Agric Sin 37:717–723
Feng J, Primomo V, Li Z, Zhang Y, Jan CC, Tulsieram L, Xu SS (2009) Physical localization and genetic mapping of the fertility restoration gene Rfo in canola (Brassica napus L.). Genome 52:401–407
FitzJohn RG, Armstrong TT, Newstrom-Lloyd LE, Wilton AD, Cochrane M (2007) Hybridisation within Brassica and allied genera: evaluation of potential for transgene escape. Euphytica 158:209–230
Fu T (1981) Production and research of rapeseed in the People’s Republic of China. Eucarpia Cruciferae Newsl 6:6–7
Galbraith DW, Lambert GM, Macas J, Dolezel J (1997) Analysis of nuclear DNA content and ploidy in higher plants. Curr Protoc Cytom 2:7.6.1–7.6.22
Hale AL, Farnham MW, Nzaramba MN, Kimbeng CA (2007) Heterosis for horticultural traits in broccoli. Theor Appl Genet 115:351–360
Heyn F (1976) Transfer of restorer genes from Raphanus to cytoplasmic male sterile Brassica napus. Eucarpia Cruciferae Newsl 1:15–16
Horisaki A, Niikura S (2008) Developmental and environmental factors affecting level of self-incompatibility response in Brassica rapa L. Sex Plant Reprod 21:123–132
Hoser-Krauze J (1987) Influence of cytoplasmic male-sterility source on some characters of cauliflower (Brassica Oleracea Var. Botritis L.). Genet Polon 28:101–108
Hu XY, Sullivan-Gilbert M, Kubik T, Kubik T, Danielson J, Hnatiuk N, Marchione W, Greene T, Thompson S (2008) Mapping of the Ogura fertility restorer gene Rfo and development of Rfo allele-specific markers in canola (Brassica napus L.). Mol Breed 22:663–674
Kamiński P (2013) Development of male sterile broccoli lines with Raphanus sativus cytoplasm and assessment of their value for breeding purposes. J Hortic Res 21:101–107
Kamiński P, Podwyszyńska M, Starzycki M, Starzycka-Korbas E (2016) Interspecific hybridisation of cytoplasmic male-sterile rapeseed with Ogura cytoplasm and Brassica rapa var. pekinensis as a method to obtain male-sterile Chinese cabbage inbred lines. Euphytica 208:519–534
Katche E, Quezada-Martinez D, Katche EI, Vasquez-Teuber P, Mason AS (2019) Interspecific hybridization for Brassica. Crop Improv Crop Breed Genet Genom 1:e190007
Kitashiba H, Nasrallah JB (2014) Self-incompatibility in Brassicaceae crops: lessons for interspecific incompatibility. Breed Sci 64:23–37
Li HT, Chen X, Yang Y, Xu JS, Gu JX, Fu J, Qian XJ, Zhang SC, Wu JS, Liu KD (2011) Development and genetic mapping of microsatellite markers from whole genome shotgun sequences in Brassica oleracea. Mol Breed 28:585–596
Li HT, Younas M, Wang XF, Li XM, Chen L, Zhao B, Chen X, Xu JS, Hou F, Hong BH (2013) Development of a core set of single-locus SSR markers for allotetraploid rapeseed (Brassica napus L.). Theor Appl Genet 126:937–947
Li QF, Zhou QH, Mei J, Zhang YJ, Li JN, Li ZY, Ge XH, Xiong ZY, Huang YJ, Qian W (2014) Improvement of Brassica napus via interspecific hybridization between B. napus and B. oleracea. Mol Breed 34:1955–1963
Li J, Zhang CL, Guan CY, Luo L, Ren L, Wei WH, Lu GY, Fang XP (2017) Analysis of intergeneric sexual hybridization between transgenic Brassica oleracea and Sinapis alba. Euphytica 213:271
Liu C, Yao X, Li G, Cai J, Xie Z (2018) Analysis of genetic diversity and population structure of Brassica oleracer based on SSR markers. Mol Plant Breed 16:1327–1337
Mao Z, Pan Y, Jin Y, Dai Z, Wang J, Zhang Z, Y-p PAN (2012) Selection and utilization of cytoplasmic male sterile line of broccoli. Jiangsu J Agric Sci 28:1511–1512
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Ogura H (1968) Studies on the new male sterility in Japanese radish, with special references on the utilization of this sterility towards the practical raising of hybrid seeds. Mem Fac Agric Kagoshima Univ 6:40–75
Pelletier G, Primard C, Ferault M, Vedel F, Chetrit P, Renard M, Delourme R (1988) Use of protoplasts on plant breeding: cytoplasmic aspects. In: Progress in plant protoplast research. Springer, pp 169–176
Prakash S, Kirti P, Bhat S, Gaikwad K, Kumar VD, Chopra V (1998) A Moricandia arvensis-based cytoplasmic male sterility and fertility restoration system in Brassica juncea. Theor Appl Genet 97:488–492
Prakash S, Bhat SR, Fu T (2009) Wild germplasm and male sterility. In: Gupta SK (ed) Biology and breeding of crucifers. CRC Press, Boca Raton
Primard-Brisset C, Poupard J, Horvais R, Eber F, Pelletier G, Renard M, Delourme R (2005) A new recombined double low restorer line for the Ogu-INRA cms in rapeseed (Brassica napus L.). Theor Appl Genet 111:736–746
Rawat D, Anand IJ (1979) Male sterility in Indian mustard Indian. J Genet Plant Breed 39:412–414
Ren WJ, Li ZY, Han FQ, Zhang B, Li X, Fang ZY, Yang LM, Zhuang M, Lv HH, Liu YM, Wang Y, Yu HL, Zhang YY (2020) Utilization of Ogura CMS germplasm with the clubroot resistance gene by fertility restoration and cytoplasm replacement in Brassica oleracea L. Hortic Res 7:61
Sharma S, Vinod (2002) Breeding for cytoplasmic male sterility in broccoli (Brassica oleracea L. var. italica Plenck). Indian J Genet Plant Breed 62:165–166
Sharma BB, Kalia P, Singh D, Sharma TR (2017) Introgression of black rot resistance from Brassica carinata to cauliflower (Brassica oleracea botrytis group) through embryo rescue. Front Plant Sci 8:1255
Shu JS, Liu YM, Li ZS, Zhang LL, Fang ZY, Yang LM, Zhuang M, Zhang YY, Lv HH (2016) Detection of the diversity of cytoplasmic male sterility sources in broccoli (Brassica oleracea var. Italica) using mitochondrial markers. Front Plant Sci 7:927
Singh K, Srivastava K (2006) Characterization of different cytoplasmic male sterility systems in Indian mustard (Brassica juncea L. Czern & Coss). Plant Breed 125:72–76
Singh S, Dey S, Bhatia R, Kumar R, Behera T (2019) Current understanding of male sterility systems in vegetable Brassicas and their exploitation in hybrid breeding. Plant Reprod 32:231–256
Tonguç M, Griffiths P (2004) Transfer of powdery mildew resistance from Brassica carinata to Brassica oleracea through embryo rescue. Plant Breeding 123:587–589
Vasanthi HR, Mukherjee S, Das DK (2009) Potential health benefits of broccoli—a chemico-biological overview. Mini Rev Med Chem 9:749–759
Wan ZJ, Jing B, Tu JX, Ma CZ, Shen JX, Yi B, Wen J, Huang T, Wang XJ, Fu TD (2008) Genetic characterization of a new cytoplasmic male sterility system (hau) in Brassica juncea and its transfer to B. napus. Theor Appl Genet 116:355–362
Wan Z, Tan Y, Shi M, Xu Y, Aryamanesh N, Yan G (2013) Interspecific introgression of male sterility from tetraploid oilseed Brassica napus to diploid vegetable B. rapa through hybridisation and backcrossing. Crop Pasture Sci 64:652–659
Wei Y, Li F, Zhang S, Zhang S, Zhang H, Sun R (2018) Characterization of interspecific hybrids between flowering Chinese cabbage and Chinese Kale. Agronomy 8:258
Yamagishi H, Bhat SR (2014) Cytoplasmic male sterility in Brassicaceae crops. Breed Sci 64:38–47
Yu HL, Fang ZY, Liu YM, Yang LM, Zhuang M, Lv HH, Li ZS, Zhang YY (2015) Genetic background screen of inter-specific hybrids between SSR marker assisted Chinese kale and rapeseed. China Veg 8:14–21
Yu HL, Fang ZY, Liu YM, Yang LM, Zhuang M, Lv HH, Li ZS, Han FQ, Liu XP, Zhang YY (2016) Development of a novel allele-specific Rfo marker and creation of Ogura CMS fertility-restored interspecific hybrids in Brassica oleracea. Theor Appl Genet 129:1625–1637
Yu HL, Li ZY, Yang LM, Liu YM, Zhuang M, Zhang LG, Lv HH, Li ZS, Han FQ, Liu XP (2017) Morphological and molecular characterization of the second backcross progenies of Ogu-CMS Chinese kale and rapeseed. Euphytica 213:55
Yu HL, Li ZY, Yang LM, Liu YM, Zhuang M, Lv HH, Li ZS, Fang ZY, Zhang YY (2018) Comparison of seed-setting and the transmission rate of Rfo during the distant hybridization of Chinese kale × Brassica napus and cabbage × B. napus. Acta Hortic Sin 45:61–70
ZhangXH Liu TJ, Li XX, Duan MM, Wang JL, Qiu Y, Wang HP, Song JP, Shen D (2016) Interspecific hybridization, polyploidization, and backcross of Brassica oleracea var. alboglabra with B. rapa var. purpurea morphologically recapitulate the evolution of Brassica vegetables. Sci Rep 6:18618
Acknowledgements
The experiment was performed at Institute of Horticulture, Shanghai Academy of Agricultural Sciences, National Center for Vegetable Improvement (Shanghai Branch), Shanghai Key Laboratory of Protected Horticultural Technology. We thank Liyong Yang from Institute of Crop Breeding and Cultivation, Shanghai Academy of Agricultural Sciences for providing the rapeseed restorer material.
Funding
This research was funded by the National Key R&D Program of China (Grant No. 2017YFD0101805), Shanghai Agriculture Applied Technology Development Program, China (Grant No. G2016060105), The Youth Talent Development Plan of Shanghai Municipal Agricultural System, China (Grant No. 20180116).
Author information
Authors and Affiliations
Contributions
Conceptualization, Z.X.; methodology, C.L.; validation, C.L., G.L. and X.Y.; formal analysis, C.L., L.H. and X.W.; resources, X.Y. and C.L.; data curation, G.L.; writing—original draft preparation, C.L.; writing—review and editing, C.L.; visualization, G.L.; supervision, Z.X.; project administration, Z.X.; funding acquisition, Z.X. All authors have read and agreed to the published version of the manuscript.”
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chun-qing Liu and Guang-qing Li have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
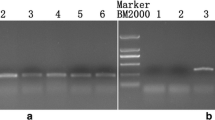
PCR amplification with the primers BnRfo-AS2F/BnRfo-AS2R (a,c) and BnRfo-AS2F/BnRFO-NEW-R (b,d). Lanes 1-22: interspecific hybrids F1 from crosses of B14 as female parents, lanes 25-32: interspecific hybrids F1 from crosses of 137 as female parents, lanes 23 and 33: parent rapeseed, lanes 24 and 34: parent broccoli. Lanes 1-11: BC1 plants from crosses of B14 as female parents, lanes 25-32: BC1 plants from crosses of 137 as female parents, lanes 12 and 33: parent rapeseed, lanes 13 and 34: parent broccoli, M: marker (PNG 338 kb)
Figure S2
PCR amplification with the primers BnRfo-AS2F/BnRfo-AS2R (a,c) and BnRfo-AS2F/BnRFO-NEW-R (b,d) (PNG 162 kb)
Figure S3
PCR amplification with the primers BnRfo-AS2F/BnRfo-AS2R (a,c) and BnRfo-AS2F/BnRFO-NEW-R (b,d)). Lanes 1-9: BC2 plants from crosses of B14 as female parents, lanes 12-27: BC2 plants from crosses of 137 as female parents, lanes 10 and 28: parent rapeseed, lanes 11 and 29: parent broccoli, M: marker (PNG 231 kb)
Rights and permissions
About this article
Cite this article
Liu, Cq., Li, Gq., Yao, Xq. et al. Characterization of Ogura CMS fertility-restored interspecific hybrids and backcross progenies from crosses between broccoli and rapeseed. Euphytica 216, 194 (2020). https://doi.org/10.1007/s10681-020-02721-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-020-02721-8