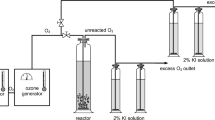
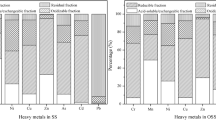
Abstract
Sulfamethoxazole (SMX) is commonly detected in wastewater and cannot be completely decomposed during conventional treatment processes. Ozone (O3) is often used in water treatment. This study explored the influence of natural organic matters (NOM) in secondary effluent of a sewage treatment plant on the ozonation pathways of SMX. The changes in NOM components during ozonation were also analyzed. SMX was primarily degraded by hydrolysis, isoxazole-ring opening, and double-bond addition, whereas hydroxylation was not the principal route given the low maximum abundances of the hydroxylated products, with m/z of 269 and 287. The hydroxylation process occurred mainly through indirect oxidation because the maximum abundances of the products reduced by about 70% after the radical quencher was added, whereas isoxazole-ring opening and double-bond addition processes mainly depended on direct oxidation, which was unaffected by the quencher. NOM mainly affected the degradation of micropollutants by consuming •OH rather than O3 molecules, resulting in the 63%–85% decrease in indirect oxidation products. The NOM in the effluent were also degraded simultaneously during ozonation, and the components with larger aromaticity were more likely degraded through direct oxidation. The dependences of the three main components of NOM in the effluent on indirect oxidation followed the sequence: humic-like substances>fluvic-like substance-s>protein-like substances. This study reveals the ozonation mechanism of SMX in secondary effluent and provides a theoretical basis for the control of SMX and its degradation products in actual water treatment.

Similar content being viewed by others
References
Augugliaro V, Bellardita M, Loddo V, Palmisano G, Palmisano L, Yurdakal S (2012). Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. Journal of Photochemistry and Photobiology C, Photochemistry Reviews, 13(3): 224–245
Bader H, Hoigné J (1981). Determination of ozone in water by the indigo method. Water Research, 15(4): 449–456
Buxton G V, Greenstock C L, Helman W P, Ross A B (1988). Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in aqueous solution. Journal of Physical and Chemical Reference Data, 17(2): 513
Chen S, Blaney L, Chen P, Deng S, Hopanna M, Bao Y, Yu G (2019). Ozonation of the 5-fluorouracil anticancer drug and its prodrug capecitabine: Reaction kinetics, oxidation mechanisms, and residual toxicity. Frontiers of Environmental Science & Engineering, 13(4): 59
Chen W, Westerhoff P, Leenheer J A, Booksh K (2003). Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environmental Science & Technology, 37(24): 5701–5710
Coble P G (1996). Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Marine Chemistry, 51(4): 325–346
Długosz M, Zmudzki P, Kwiecien A, Szczubialka K, Krzek J, Nowakowska M (2015). Photocatalytic degradation of sulfamethoxazole in aqueous solution using a floating TiO2-expanded perlite photocatalyst. Journal of Hazardous Materials, 298: 146–153
Du J, Guo W, Wang H, Yin R, Zheng H, Feng X, Che D, Ren N (2018). Hydroxyl radical dominated degradation of aquatic sulfamethoxazole by Fe0/bisulfite/O2: Kinetics, mechanisms, and pathways. Water Research, 138: 323–332
Gómez-Ramos M del MMezcua M, Aguera A, Fernandez-Alba A R, Gonzalo S, Rodriguez A, Rosal R (2011). Chemical and toxicological evolution of the antibiotic sulfamethoxazole under ozone treatment in water solution. Journal of Hazardous Materials, 192(1): 18–25
Graça C A L, Lima R B, Pereira M F R, Silva A M T, Ferreira A (2020). Intensification of the ozone-water mass transfer in an oscillatory flow reactor with innovative design of periodic constrictions: Optimization and application in ozonation water treatment. Chemical Engineering Journal, 389: 124412
Guo W Q, Yin R L, Zhou X J, Du J S, Cao H O, Yang S S, Ren N Q (2015). Sulfamethoxazole degradation by ultrasound/ozone oxidation process in water: Kinetics, mechanisms, and pathways. Ultrasonics Sonochemistry, 22: 182–187
Hai H, Xing X, Li S, Xia S, Xia J (2020). Electrochemical oxidation of sulfamethoxazole in BDD anode system: Degradation kinetics, mechanisms and toxicity evaluation. Science of the Total Environment, 738: 139909
Ho L, Newcombe G, Croué J P (2002). Influence of the character of NOM on the ozonation of MIB and geosmin. Water Research, 36(3): 511–518
Ioannidi A, Oulego P, Collado S, Petala A, Arniella V, Frontistis Z, Angelopoulos G N, Diaz M, Mantzavinos D (2020). Persulfate activation by modified red mud for the oxidation of antibiotic sulfamethoxazole in water. Journal of Environmental Management, 270: 110820
Jiao Y, Zhao Y, Chen Y, Wu G, Liu W, Tian X, Zheng Y (2018). Characterizing the interaction of sulfamethazine and macrophytes-derived dissolved organic matter by fluorescence spectroscopy. Environmental Science & Technology, 41(3): 8–14
Khan A H, Khan N A, Ahmed S, Dhingra A, Singh C P, Khan S U, Mohammadi A A, Changani F, Yousefi M, Alam S, Vambol S, Vambol V, Khursheed A, Ali I (2020a). Application of advanced oxidation processes followed by different treatment technologies for hospital wastewater treatment. Journal of Cleaner Production, 269: 122411
Khan N A, Ahmed S, Farooqi I H, Ali I, Vambol V, Changani F, Yousefi M, Vambol S, Khan S U, Khan A H (2020b). Occurrence, sources and conventional treatment techniques for various antibiotics present in hospital wastewaters: A critical review. Trends in Analytical Chemistry, 129: 115921
Khan N A, Khan S U, Ahmed S, Farooqi I H, Yousefi M, Mohammadi A A, Changani F (2020c). Recent trends in disposal and treatment technologies of emerging-pollutants-A critical review. Trends in Analytical Chemistry, 122: 115744
Kong S, Zhao Y G, Guo L, Gao M, Jin C, She Z (2020). Transcriptomics of Planococcus kocurii O516 reveals the degrading metabolism of sulfamethoxazole in marine aquaculture wastewater. Environmental Pollution, 265: 114939
Lai L, Yan J, Li J, Lai B (2018). Co/Al2O3-EPM as peroxymonosulfate activator for sulfamethoxazole removal: Performance, biotoxicity, degradation pathways and mechanism. Chemical Engineering Journal, 343: 676–688
Lee C Y, Lee Y (2007). Impact of water quality on the formation of bromate and formaldehyde during water ozonation. Korean Journal of Environmental Health, 33(5): 441–450
Li H, Li T, He S, Zhou J, Wang T, Zhu L (2020a). Efficient degradation of antibiotics by non-thermal discharge plasma: Highlight the impacts of molecular structures and degradation pathways. Chemical Engineering Journal, 395: 125091
Li S, Hu J (2018). Transformation products formation of ciprofloxacin in UVA/LED and UVA/LED/TiO2 systems: Impact of natural organic matter characteristics. Water Research, 132: 320–330
Li Y, Li J, Pan Y, Xiong Z, Yao G, Xie R, Lai B (2020b). Peroxymonosulfate activation on FeCo2S4 modified g-C3N4 (FeCo2S4-CN): Mechanism of singlet oxygen evolution for nonradical efficient degradation of sulfamethoxazole. Chemical Engineering Journal, 384: 123361
Li Y, Zhao X, Yan Y, Yan J, Pan Y, Zhang Y, Lai B (2019). Enhanced sulfamethoxazole degradation by peroxymonosulfate activation with sulfide-modified microscale zero-valent iron (S-mFe0): Performance, mechanisms, and the role of sulfur species. Chemical Engineering Journal, 376: 121302
Liu F, Zhou H, Pan Z, Liu Y, Yao G, Guo Y, Lai B (2020). Degradation of sulfamethoxazole by cobalt-nickel powder composite catalyst coupled with peroxymonosulfate: Performance, degradation pathways and mechanistic consideration. Journal of Hazardous Materials, 400: 123322
Liu X, Garoma T, Chen Z, Wang L, Wu Y (2012). SMX degradation by ozonation and UV radiation: a kinetic study. Chemosphere, 87(10): 1134–1140
Mao Y, Dong H, Liu S, Zhang L, Qiang Z (2020). Accelerated oxidation of iopamidol by ozone/peroxymonosulfate (O3/PMS) process: Kinetics, mechanism, and simultaneous reduction of iodinated disinfection by-product formation potential. Water Research, 173: 115615
McKnight D M, Andrews E D, Spaulding S A, Aiken G R (1994). Aquatic fulvic acids in algal-rich antarctic ponds. Limnology and Oceanography, 39(8): 1972–1979
Milh H, Schoenaers B, Stesmans A, Cabooter D, Dewil R (2020). Degradation of sulfamethoxazole by heat-activated persulfate oxidation: Elucidation of the degradation mechanism and influence of process parameters. Chemical Engineering Journal, 379: 122234
Milori D M B P, Martin-Neto L, Bayer C, Mielniczuk J, Bagnato V S (2002). Humification degree of soil humic acids determined by fluorescence spectroscopy. Soil Science, 167(11): 739–749
Ninwiwek N, Hongsawat P, Punyapalakul P, Prarat P (2019). Removal of the antibiotic sulfamethoxazole from environmental water by mesoporous silica-magnetic graphene oxide nanocomposite technology: Adsorption characteristics, coadsorption and uptake mechanism. Colloids and Surfaces. A, Physicochemical and Engineering Aspects, 580: 123716
Niu J, Zhang L, Li Y, Zhao J, Lv S, Xiao K (2013). Effects of environmental factors on sulfamethoxazole photodegradation under simulated sunlight irradiation: Kinetics and mechanism. Journal of Environmental Sciences-China, 25(6): 1098–1106
Qian Y, Liu X, Li K, Gao P, Chen J, Liu Z, Zhou X, Zhang Y, Chen H, Li X, Xue G (2020). Enhanced degradation of cephalosporin antibiotics by matrix components during thermally activated persulfate oxidation process. Chemical Engineering Journal, 384: 123332
Ren M, Drosos M, Frimmel F H (2018). Inhibitory effect of NOM in photocatalysis process: Explanation and resolution. Chemical Engineering Journal, 334: 968–975
Senesi N, D’orazio V, Ricca G (2003). Humic acids in the first generation of EUROSOILS. Geoderma, 116(3–4): 325–344
Shahmahdi N, Dehghanzadeh R, Aslani H, Bakht Shokouhi S (2020). Performance evaluation of waste iron shavings (Fe0) for catalytic ozonation in removal of sulfamethoxazole from municipal waste-water treatment plant effluent in a batch mode pilot plant. Chemical Engineering Journal, 383: 123093
Trovó A G, Nogueira R F, Aguera A, Fernandez-Alba A R, Sirtori C, Malato S (2009). Degradation of sulfamethoxazole in water by solar photo-Fenton. Chemical and toxicological evaluation. Water Research, 43(16): 3922–3931
Wang H, Mustafa M, Yu G, Ostman M, Cheng Y, Wang Y, Tysklind M (2019). Oxidation of emerging biocides and antibiotics in wastewater by ozonation and the electro-peroxone process. Chemosphere, 235: 575–585
Wang J, Chu L (2016). Irradiation treatment of pharmaceutical and personal care products (PPCPs) in water and wastewater: An overview. Radiation Physics and Chemistry, 125: 56–64
Wang J, Zhuan R (2020). Degradation of antibiotics by advanced oxidation processes: An overview. Science of the Total Environment, 701: 135023 doi:https://doi.org/10.1016/j.scitotenv.2019.135023
Westerhoff P, Aiken G, Amy G, Debroux J (1999). Relationships between the structure of natural organic matter and its reactivity towards molecular ozone and hydroxyl radicals. Water Research, 33(10): 2265–2276
Willach S, Lutze H V, Eckey K, Loppenberg K, Luling M, Terhalle J, Wolbert J B, Jochmann M A, Karst U, Schmidt T C (2017). Degradation of sulfamethoxazole using ozone and chlorine dioxide-Compound-specific stable isotope analysis, transformation product analysis and mechanistic aspects. Water Research, 122: 280–289
Xiao K, Yu J, Wang S, Du J, Tan J, Xue K, Wang Y, Huang X (2020). Relationship between fluorescence excitation-emission matrix properties and the relative degree of DOM hydrophobicity in wastewater treatment effluents. Chemosphere, 254: 126830
Yang C C, Huang C L, Cheng T C, Lai H T (2015). Inhibitory effect of salinity on the photocatalytic degradation of three sulfonamide antibiotics. International Biodeterioration & Biodegradation, 102: 116–125
Ye B, Chen Z, Li X, Liu J, Wu Q, Yang C, Hu H, Wang R (2019). Inhibition of bromate formation by reduced graphene oxide supported cerium dioxide during ozonation of bromide-containing water. Frontiers of Environmental Science & Engineering, 13(6): 86
Yu H, Qu F, Zhang X, Shao S, Rong H, Liang H, Bai L, Ma J (2019). Development of correlation spectroscopy (COS) method for analyzing fluorescence excitation emission matrix (EEM): A case study of effluent organic matter (EfOM) ozonation. Chemosphere, 228: 35–43
Yuan R, Zhu Y, Zhou B, Hu J (2019). Photocatalytic oxidation of sulfamethoxazole in the presence of TiO2: Effect of matrix in aqueous solution on decomposition mechanisms. Chemical Engineering Journal, 359: 1527–1536
Zhang H, Wang Z, Li R, Guo J, Li Y, Zhu J, Xie X (2017a). TiO2 supported on reed straw biochar as an adsorptive and photocatalytic composite for the efficient degradation of sulfamethoxazole in aqueous matrices. Chemosphere, 185: 351–360
Zhang S, Yu G, Chen J, Zhao Q, Zhang X, Wang B, Huang J, Deng S, Wang Y (2017b). Elucidating ozonation mechanisms of organic micropollutants based on DFT calculations: Taking sulfamethoxazole as a case. Environmental Pollution, 220: 971–980
Zhang T, Tao Y Z, Yang H W, Chen Z, Wang X M, Xie Y F (2020). Study on the removal of aesthetic indicators by ozone during advanced treatment of water reuse. Journal of Water Process Engineering, 36: 101381
Zhao X, Wu Y, Zhang X, Tong X, Yu T, Wang Y, Ikuno N, Ishii K, Hu H (2019). Ozonation as an efficient pretreatment method to alleviate reverse osmosis membrane fouling caused by complexes of humic acid and calcium ion. Frontiers of Environmental Science & Engineering, 13(4): 55
Zhou J, Wang J J, Baudon A, Chow A T (2013). Improved fluorescence excitation-emission matrix regional integration to quantify spectra for fluorescent dissolved organic matter. Journal of Environmental Quality, 42(3): 925–930
Acknowledgements
This work was supported by the National Key Research and Development Project (No. 2019YFD1100204). The experimental supporting by National Environmental and Energy Base for International Science & Technology Cooperation was greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• SMX was mainly degraded by hydrolysis, isoxazole oxidation and double-bond addition.
• Isoxazole oxidation and bond addition products were formed by direct ozonation.
• Hydroxylated products were produced by indirect oxidation.
• NOM mainly affected the degradation of SMX by consuming •OH rather than O3.
• Inhibitory effect of NOM on SMX removal was related to the components’ aromaticity.
Supplementary Materials
Rights and permissions
About this article
Cite this article
Liu, X., Su, X., Tian, S. et al. Mechanisms for simultaneous ozonation of sulfamethoxazole and natural organic matters in secondary effluent from sewage treatment plant. Front. Environ. Sci. Eng. 15, 75 (2021). https://doi.org/10.1007/s11783-020-1368-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11783-020-1368-0