Abstract
Leptocephalus larvae have transparent bodies with tubular intestines that usually lack identifiable food items when they are collected, so mystery has surrounded efforts to determine what they feed on. Artificially spawned and reared first-feeding larvae were found to be highly selective in what they would eat, but they would consume rotifers and eventually ate specially formulated diets that contained shark egg yolk. Gut content studies on wild-caught leptocephali in the Atlantic and Pacific observed marine snow-associated materials such as discarded appendicularian houses, zooplankton fecal pellets, protists, and amorphous materials, and DNA sequencing indicated that the gut contents contain materials originating from a wide range of microorganisms and food web zooplankton species that were likely consumed in marine snow. Isotopic studies found a low trophic position of leptocephali and inter-taxa and geographic signature differences. Behavioral studies with leptocephali and the characteristics and size-scaling of the teeth are also consistent with feeding on marine snow-related particles. The feeding strategy of leptocephali appears to be based on consuming types of marine snow that contain nutritious and easily assimilated carbohydrates, fatty acids, and other materials that facilitate rapid conversion to glycosaminoglycans and tissues for energy storage and growth.
Similar content being viewed by others
Introduction
Leptocephali are a unique type of fish larvae that are present in the upper few hundred meters throughout the world’s oceans from tropical to temperate latitudes (Smith 1989; Miller 2009). They are the larvae of anguilliform fishes and their close relatives within the Elopomorpha, which live in a wide range of habitats from coastal areas to deep-benthic environments and in the meso- and bathypelagic zones (Miller and Tsukamoto 2004). The larvae, however, are all present together in the upper 300 m offshore, most are present in the upper 100 m at night, and some use diel vertical migration (see Miller and Tsukamoto 2020). They are specialized for extreme transparency and accumulation of energy storage compounds in an internal gelatinous matrix, and this makes them have a minimal amount of body tissues (Smith 1989; Pfeiler 1999; Miller 2009). They grow to large sizes because their transparency and other behavioral adaptations likely reduce predation (see Miller and Tsukamoto 2020).
Their unusual body form allows them to have low respiration rates (Pfeiler and Govoni 1993; Bishop and Torres 1999), osmolarity close to seawater (Hulet and Robins 1989), and buoyancy that is regulated by chloride cells (Tsukamoto et al. 2009). As they grow, glycosaminoglycans (GAG) such as hyaluronan (Pfeiler et al. 2002; Okamura et al. 2018) and other compounds such as fatty acids (Deibel et al. 2012; Liénart et al. 2016) are stored in the high-water-content mucinous pouch that fills most of their bodies (Donnelly et al. 1995; Pfeiler 1999; Bishop et al. 2000). These materials are converted to new tissues during metamorphosis into the juvenile stage.
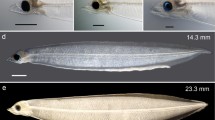
Leptocephali of anguilliform families also have a wide range of body shapes (Miller and Tsukamoto 2004, 2020; Miller 2009) and head and jaw shapes (Fig. 1). Their heads and jaws range from being very long and pointed (Fig. 1c, h, j) to short and rounded (Fig. 1b, g, l). Some jaw shapes such as those of Eurypharynx pelecanoides (Fig. 2c), which are the larvae of gulper eels, seem to be linked to their juvenile and adult jaw shapes. A different type of jaw and tooth structure is present in Congriscus larvae (Fig. 1d) that grow to large sizes.
Photographs of the heads of freshly caught leptocephali that illustrate the wide range of jaw shapes and eyes and their proportional sizes to the head regions of 30 mm Anguilla marmorata (a), 58 mm Muraenidae (b), 40 mm Gnathophis-type (c), 124 mm Congriscus (formerly referred to as Thallassenchelys) (Congriscus is currently within the Congridae) (d), 144 mm Nemichthys (Nemichthyidae) (e), 51 mm Kaupichthys (Chlopsidae) (f), 132 mm Ophichthidae (g), 53 mm Muraenidae (h), 151 mm Bathycongrus (Congridae) (i), 225 mm Ariosoma-type (Congridae) (j), 62 mm Illyophinae (Synaphobranchidae) (k), and 57 mm Synaphobranchinae (Synaphobranchidae) (l) leptocephali. Scale bars are 1 mm

Modified from Miller (2009)
The heads and jaws of freshly caught small leptocephali that show the long forward-pointing teeth of a 7 mm Anguilla marmorata collected along the West Mariana Ridge (a), an 8.2 mm Muraenidae collected near Suruga Seamount of the West Mariana Ridge (b), and an 8.8 mm Eurypharynx pelecanoides (c).
What this diversity of different sizes and shapes of leptocephali feed on was slow to be discovered because of a lack of identifiable materials in their intestines. Most fish larvae feed on zooplankton such as copepods (Hunter 1981), so because no zooplankton were ever seen in leptocephalus guts, a hypothesis was proposed that leptocephali do not feed, and instead absorb dissolved organic matter (DOM) directly across their skin (Pfeiler 1986, 1999). That hypothesis was disproved by observations of gut contents of leptocephali (Otake et al. 1993; Mochioka and Iwamizu 1996) and observations of leptocephali feeding in the laboratory (Tanaka et al. 1995; Mochioka et al. 1993). Based on observations of leptocephalus gut contents and other types of information, it was eventually realized that they seem to feed on the type of particulate organic matter (POM) referred to as marine snow (Miller et al. 2011, 2013). Marine snow is defined as being aggregates of organic detritus, microorganisms, and inorganic clay minerals, so it is comprised of materials that originate from zooplankton, phytoplankton, and exudates produced by biological communities (Alldredge and Silver 1988; Cowen and Holloway 1996), which would usually appear to be unidentifiable amorphous food material when present in leptocephalus intestines.
Understanding what leptocephali eat was a critical objective of efforts to produce glass eel seedlings for commercial aquaculture by rearing larvae produced from artificial spawning of adult eels in the laboratory, and these techniques were pioneered using Japanese eels, Anguilla japonica (Tanaka et al. 2001, 2003; Okamura et al. 2014; Tanaka 2015). Spawning protocols were developed using hormone injections (see Okamura et al. 2014; Tanaka 2015), but finding what the newly hatched larvae would feed on was more difficult. They would consume rotifers (Tanaka et al. 1995), but a paste-like diet including shark egg yolk was eventually formulated that leptocephali would eat and show growth (Tanaka et al. 2003, 2006). Having leptocephali being hatched and reared in the laboratory also enabled studies on their digestive systems (Otake 1996; Pedersen et al. 2003; Kurokawa et al. 1995, 1996, 2011) and a variety of other studies. Recent research has also used artificially reared first-feeding European eel larvae, Anguilla anguilla (Sørensen et al. 2016) to see what they will eat (Butts et al. 2016) and to study the functional morphology of their head and jaws (Bouilliart et al. 2015).
The artificial diet fed to young eel larvae did not appear similar to what leptocephali must be eating in the ocean, however, so research continued to understand their natural diets. Direct observations of gut contents of leptocephali from coastal Japan found discarded appendicularian houses and zooplankton fecal pellets (technically considered marine snow) and amorphous material (Otake et al. 1993; Mochioka and Iwamizu 1996). Amorphous materials and ciliates were observed in the intestines of other coastal leptocephali (Govoni 2010), and observations of leptocephalus gut contents were also made with larvae collected in offshore areas (Miller et al. 2011, 2019; Tomoda et al. 2018). Artificially reared larvae were observed to ingest marine snow-related materials (Tomoda et al. 2015; Chow et al. 2017).
The observations of identifiable objects linked to marine snow aggregates indicated that consumption of marine snow particles occurs, but it did not explain what else might be consumed or what parts of marine snow might be digested and assimilated. The wide range of head and jaw shapes (Fig. 1) made it unclear whether all leptocephali ate the same things, and the function of the forward-pointing teeth of small leptocephali (Fig. 2) was uncertain.
Stable isotope studies were also used to explore the feeding ecology of leptocephali, with the first studies being conducted in the western North Pacific (Otake et al. 1993; Kimura and Tsukamoto 2006). More recent studies compared the signatures of leptocephali to POM from the same area as the larvae were collected (Miyazaki et al. 2011; Liénart et al. 2016; Quattrini et al. 2019) and also to other food-web components such as zooplankton (Feunteun et al. 2015; Ghinter et al. 2020). The findings of these studies were generally consistent with the larvae feeding on marine snow, but they also found that there appeared to be two taxonomic groups of leptocephali with different isotopic signatures, and geographic variations in signatures were detected.
DNA sequence analysis of the gut contents of small European eel leptocephali that were collected in their Sargasso Sea spawning area found a wide range of taxonomic groups were present in the gut contents (Riemann et al. 2010; Ayala et al. 2018). The frequent presence of DNA of gelatinous zooplankton such as siphonophores was used to suggest that those species may be important in the diets of the leptocephali (Riemann et al. 2011; Ayala et al. 2018). DNA from siphonophores and other taxonomic groups was also found to be present in the gut contents of leptocephali in the western North Pacific (Chow et al. 2019). Siphonophore tentacles or other small life history stages may contribute to marine snow aggregation or be consumed directly by leptocephali, which seems to explain their detection in the gut contents of leptocephali (Miller et al. 2019, 2020).
Marine snow is widely present in the ocean and is usually most abundant within the upper 150 m in offshore areas (Hebel and Karl 2001; Pilskaln et al. 2005; Munk et al. 2018), which overlaps with the depths where leptocephali typically occur (Castonguay and McCleave 1987). Many types of materials aggregate into marine snow (Alldredge and Silver 1988; Shanks and Walters 1997; Kiørboe 2000), and carbohydrates in the form of transparent exoparticles (TEP) (Alldredge et al. 1993; Passow 2002; Engel 2004; Mari et al. 2017) help to make materials stick together. Therefore, marine snow can contain a wide range of materials that reflect the biological activity in each area, and leptocephali appear to feed on and obtain nutrition from some of these materials. It has also recently been realized that marine snow can contain single-celled thraustochytrid protists of the Labyrinthulomycetes (Lyons et al. 2005; Li et al. 2013; Bochdansky et al. 2017), which are heterotrophic organisms that are recently being found to be widely distributed in the ocean (Raghukumar 2002; Marchan et al. 2018). A recent study observed the presence of round cells that resembled those thraustochytrid protists in gut contents of leptocephalus intestines in the Sargasso Sea along with many other types of materials (Miller et al. 2019).
The objective of this review is to provide a broad up-to-date overview of what is known about the food of leptocephali in nature and in aquaculture rearing conditions to help evaluate the feeding ecology of leptocephali. We overview the history of hypotheses about what leptocephali eat, the history and present state of efforts to feed larvae for aquaculture, and then examine what has been learned about the food and isotopic composition of these unusual larvae. This can facilitate further research on their feeding ecology in the ocean and for aquaculture.
Hypotheses for leptocephalus food sources
Direct absorption of DOM
The general lack of observable gut contents and other factors were used by Pfeiler (1986) to formulate a hypothesis that leptocephali might obtain nutrition by directly absorbing DOM across their skin. Most evidence supporting the hypothesis was derived from the detailed morphological study of Ariosoma balearicum by Hulet (1978). That study reported that the digestive system was not fully developed in leptocephali before metamorphosis and the intestine may not be fully open. Another important point was that the body surface epithelium was only 2–3 cell layers thick, and the outer layer of cells had numerous filamentous projections. The projections were compared to intestinal microvilli, which might allow sodium-mediated uptake of DOM across the epithelium. The large surface-to-volume ratio of the laterally compressed leptocephalus bodies was also a key point making direct absorption possible. The direct DOM absorption hypothesis continued to be considered as a possibility by Pfeiler (1999), even after leptocephalus gut contents were examined (Otake et al. 1993; Mochioka and Iwamizu 1996), leptocephali were observed to consume rotifers (Tanaka et al. 1995), and late-stage Muraenesocidae leptocephali were observed to eat squid paste (Mochioka et al. 1993). It was suggested that ingested food sources may be insufficient because leptocephali did not survive very long in the laboratory, so absorption of DOM might be required (Pfeiler 1999).
This hypothesis was never clearly tested, but the successful rearing of Japanese eel larvae in captivity and further observations of gut contents indicated that direct DOM absorption was not nutritionally important for leptocephali. Pfeiler (1986) also pointed out that the large surface-to-volume ratio and filamentous projections would be important for oxygen uptake by leptocephali, which lack gills until near metamorphosis. Whether or not they can also absorb DOM across their skin remains to be determined.
Feeding on POM and intestinal absorption of DOM
Most collections of anguilliform leptocephali were conducted offshore in the low-productivity subtropical gyres where anguillid eels spawn, so few distinctive gut contents were observed. However, leptocephali caught as bycatch of various types of fisheries for other small fishes in coastal areas of the Seto Inland Sea of Japan contained visible POM objects. Otake et al. (1993) examined 613 leptocephali of Conger myriaster, C. japonicus, and Muraenesox cinereus caught in sand eel or sardine fishing in the upper 20 m in coastal waters to study their gut contents. The observations found from 1 to 31 zooplankton fecal pellets (100–250 μm) in the intestines of 105 of the larvae and smaller objects < 20 μm were in more than 90% of the 613 examined leptocephali. The objects included fecal pellets apparently from appendicularians and copepods and smaller amorphous materials consisting of smaller particle aggregates. Various types of fecal pellets (Figs. 3 and 4) continue to be seen in leptocephalus gut contents, many of which are shaped like those of appendicularians (Fig. 4; Miller et al. 2011, 2019). Fecal pellets of other types of zooplankton and amorphous materials were present in the guts of leptocephali collected in the semi-enclosed Tomini Bay of Sulawesi Island, Indonesia (Fig. 3; Miller et al. 2011).

(modified from Miller et al. 2011)
The head region (a), gut contents, including zooplankton fecal pellets, beginning to exude out of the intestine (b), and view of the contents farther out (c) of a 66 mm Chlopsidae leptocephalus collected in Tomini Bay of Sulawesi Island
Photographs of gut content objects within leptocephalus intestines that appear to be an appendicularian house with one of its fecal pellets in the intestine of a 56 mm Muraenidae larva (a), oval objects possibly with tentacle materials in the intestine of a 197 mm Avocettina (Nemichthyidae) leptocephalus, which look more irregularly shaped compared to appendicularian houses (also no fecal pellets) and might be very small siphonophore life history stages (b), fecal pellets and various unidentifiable objects in a different part of the intestine of the same larva (c), and gut content materials that have flowed out of the intestines of a 23 mm Megalopidae (Elopiformes) larva (d), a 61 mm Serrivomeridae larva including remnants of a likely appendicularian house (e), and a 35 mm Derichthys serpentinus larva (f) that includes many round objects and some fecal pellets. No scale bar is available for (f), which is likely within the magnification range of (d) and (e)
Histological tracer techniques were also used to examine the structure and function of the intestines of Japanese eel and M. cinereus leptocephali to look for clues about their food sources. Well-developed lamellar membranous structures were observed in the midgut absorptive cells of Conger leptocephali that might function in the absorption of dissolved organic matter contained in seawater (Otake et al. 1993). Similar structures were observed in the midgut of Japanese eel and pike eel leptocephali (Otake et al. 1995; Otake 1996). The structure of the hindgut appears to be functional for uptake and intracellular digestion of intact macromolecules (Otake 1996), and uptake of protein macromolecules has been shown there (Otake et al. 1995). The structures of the midgut of leptocephali suggested they may be able to assimilate DOM in water that is ingested along with food particles even before the digestive process is complete. This seems possible since some DOM is present within marine snow particles (Alldredge 2000).
Consumption of appendicularian houses
Mochioka and Iwamizu (1996) also used leptocephali mostly from coastal fisheries in Japan, and examined the gut contents of 234 specimens of eight species of leptocephali (Congridae, Muraenesocidae, Muraenidae, Nettastomatidae, Ophichthidae). It was found that 80% of the guts of larvae that had been feeding contained appendicularian houses, and 56% contained appendicularian fecal pellets. The shapes of fecal pellets of other types of zooplankton are different (Wilson et al. 2008), and 17% of the larvae in the study of Mochioka and Iwamizu (1996) had also consumed other types of fecal pellets. Discarded appendicularian houses typically contain some of their own fecal pellets (Taguchi 1982; Alldredge and Sliver 1988), so the leptocephali appeared to have been ingesting discarded houses that also contained their fecal pellets.
Later studies and recent observations also showed the presence of appendicularian houses in the gut contents of leptocephali in the western Pacific, Tomini Bay in the Indonesian Seas, and in the Sargasso Sea (Fig. 4a; Miller et al. 2011, 2019). These houses appear as translucent oval objects that usually have fecal pellets associated with them (Fig. 4a). When they have been removed and examined, their house shapes and filters were seen (Mochioka and Iwamizu 1996; Miller et al. 2019).
The houses are discarded frequently as soon as their filters become clogged, and a new pre-formed house is immediately inflated to allow filter feeding to resume by the appendicularian (Sato et al. 2003). The filters might contain digestible organismal materials, and the discarded houses are colonized by various organisms, which makes them a food source for various types of zooplankton or fish larvae (Alldredge 1976), but it is unknown whether they contribute to the nutrition of leptocephali.
Feeding on marine snow
Appendicularian houses and fecal pellets are considered to be components of marine snow (Alldredge and Silver 1988), so it became apparent that leptocephali are probably feeding on marine snow particles. Amorphous materials consistent with marine snow, zooplankton fecal pellets, and discarded appendicularian houses were then shown clearly in photographs of gut contents of a variety of species of leptocephali (Miller et al. 2011), and similar types of materials continue to be seen especially in larvae collected during the daytime (Fig. 4; Miller et al. 2019). Amorphous material was also observed in the intestines of M. cinereus leptocephali captured near tropical coral reefs (Miller et al. 2010). One leptocephalus has also been observed to have consumed what appears to be reddish-colored marine snow (Fig. 5), which could have come from materials released by a bloom of red-pigmented phytoplankton. Exactly what this amorphous material in leptocephalus intestines was comprised of had not been directly determined, or validated to have come from marine snow. But when it was analyzed for DNA sequences using the gut contents of small European eel larvae (Ayala et al. 2018) or those of more species of larger leptocephali (Chow et al. 2019), the sequences of many marine taxa were found to be present, and microorganisms could be seen under higher magnifications (Miller et al. 2019) which were consistent with a marine snow origin.
The head and anterior body of a 31 mm Muraenidae leptocephalus with reddish gut contents (a, b) that was collected in the North Equatorial Current region (5°N, 136°E) of the western North Pacific (January 2013), and close-up views of the reddish-colored amorphous gut contents within the end of its esophagus (c) and inside its intestine (d)
Another way to evaluate whether leptocephali obtain their nutrition from marine snow is to determine their trophic position within the food web where they are collected. The relatively new technique of analyzing the amino acid isotope signatures of consumers is able to determine trophic positions independent of analyzing the primary producers (Chikaraishi et al. 2009). A study using amino acid isotopes with both wild-caught and hatchery-reared Japanese eel leptocephali (15–18 mm) was able to determine the trophic level of the wild leptocephali to be 2.4 (Fig. 6), a position intermediate between primary producers and primary consumers (Miller et al. 2013). In contrast, laboratory-reared larvae that were fed artificial diets containing shark egg yolk had much higher trophic positions. Because of the small size of phytoplankton and small heterotrophic microorganisms that cannot be consumed individually, the only way leptocephali could have such a low trophic position would be by feeding on marine snow that contains a mixture of materials from phytoplankton, microorganisms, and any kind of detrital material produced within the food web. That study and the observational studies on gut contents provided strong evidence that leptocephali feed on marine snow and that it could be their main food source.
The trophic position of wild-caught and aquaculture-reared Japanese eel, Anguilla japonica, leptocephali compared to various other types of marine organisms that were determined using amino acid isotopic ratios (lines in upper and lower right corners show original x- and y-axis angles) and the hypothetical position of the marine snow food of leptocephali. The natural leptocephali have a trophic position that is consistent with feeding on marine snow, but the cultured leptocephali were fed two different diets that both contained shark egg yolk, causing them to have higher trophic positions. Redrawn from Miller et al. (2013)
Feeding on gelatinous zooplankton
Westerberg (1990) had hypothesized that the teeth structure of leptocephali is adapted for grasping gelatinous objects. Then a new research approach using DNA sequencing of the gut contents of small European eel leptocephali (< 20 mm) in their Sargasso Sea spawning area detected the sequences of gelatinous zooplankton. Riemann et al. (2011) used the detection of hydrozoan 18S rRNA gene sequences in the gut contents to infer that gelatinous zooplankton might be an important component of the leptocephalus diet. That study detected 75 sequences of 17 taxonomic lineages within the intestines of 42 of the 61 examined larvae. Hydrozoan sequences (siphonophores and hydromedusae) were detected in 55% of the larvae with sequences, and sequences of the Polycystinea (radiolarians) were detected in 40% of the larvae with sequences (Riemann et al. 2011). There were also sequences of fungi, algae, dinoflagellates, crustaceans, chaetognaths, and salps. That short paper did not discuss how the leptocephali might feed on the hydrozoans, and no observations of the gut contents themselves were made.
The idea of feeding on gelatinous zooplankton was further supported in the more comprehensive study by Ayala et al. (2018) that used next-generation 18S rRNA gene sequencing (NGS) of the gut contents of small European eel larvae, in which hydrozoan sequences were detected in the gut contents of all 75 examined larvae. The proportion of sequence fragments (reads) of hydrozoans was also very high (73.5% of total number of reads) compared to other taxonomic groups, with the next highest proportion being of crustaceans (7%). The majority (75%) of the hydrozoan sequences belonged to calycophoran siphonophores. These small siphonophores are widespread species in the world’s oceans including in subtropical gyres (see Mapstone 2014) such as the Sargasso Sea (Lüskow et al. 2019). They feed using extensive tentacle arrays and have reproductive stages that die after releasing their eggs, so they have the potential to contribute to marine snow aggregates (see Miller et al. 2020). Objects that could have been siphonophore tentacles or parts of exoskeletons were observed in the gut contents of a gulper eel (Eurypharynx pelecanoides) leptocephalus (Miller et al. 2019, 2020). Slightly pigmented oval objects that appear different than appendicularian houses, such as those seen in Fig. 4b, have the potential to be small siphonophore materials (contain no fecal pellets; include possible tentacles), and a few of these objects have been observed previously (Miller et al. 2011, 2019). However, the detected siphonophore DNA was apparently contained in amorphous gut contents, because Ayala et al. (2018) reported that the gut contents they analyzed contained no visibly identifiable objects.
A wide range of other taxonomic groups from 16 eukaryotic taxonomic lineages were detected using the NGS method, which included fungi, radiolarians, dinoflagellates, chaetognaths, crustaceans (euphausiids and copepods), and fish (Ayala et al. 2018). That study also used NGS on 31 marine snow particles and found them to contain 13 eukaryotic lineages, with the highest proportions of reads being from crustaceans, followed by hydrozoans (detected in more than 20 marine snow particles) and radiolarians. The different proportions of reads between the marine snow particles and larval gut contents led the authors to evaluate whether it was energetically possible that the leptocephali might feed directly on calycophoran siphonophores and obtain much of their nutrition that way. The calculations indicated it would be possible to meet their daily nutritional needs if the leptocephali (15 mm) capture, tear apart, and eat five siphonophores per day (average size 5 mm).
This scenario does not appear to be likely, however, because the forward-pointing teeth of small leptocephali (Figs. 1 and 2) do not appear to be designed to cut into a siphonophore body (Westerberg 1990; Miller et al. 2019). So, as discussed in previous studies, other mechanisms of gelatinous zooplankton tissue entering the gut contents appear more likely (Lüskow et al. 2019; Miller et al. 2020), and the wide range of microorganisms detected in the leptocephalus gut contents could only be ingested by eating marine snow, as previously noted (Feunteun et al. 2015; Miller et al. 2019, 2020).
One possible reason for the different taxonomic composition of the larval gut contents and marine snow (Ayala et al. 2018) is that the 1–10 mm size of the marine snow particles they analyzed (Lundgreen et al. 2019) are likely larger than those consumed by the leptocephali (Miller et al. 2020). Another possible factor is that some of the DNA sequences reported by Ayala et al. (2018) could have come from the external surfaces of whole-intestine samples that were analyzed. Chow et al. (2019) tested the sequence content of the external body surface of leptocephali and found that cnidarian sequences (Hydrozoa, Anthozoa) were the most abundant taxonomic group in the body samples. Cnidarians were also the most abundant taxa in the gut contents (Fig. 7a), confirming their presence in materials consumed by leptocephali (Chow et al. 2019), but the body samples suggest that some of the hydrozoa and other sequences in the Ayala et al. (2018) study could have come from external contamination from inside the codend of the net. This might have made hydrozoan sequences appear more abundant in the gut contents, and other factors such as NGS amplification biases from hydrozoans possibly having higher rRNA gene copy numbers also need to be evaluated to understand the contribution of hydrozoans, and particularly calycophoran siphonophores, to leptocephalus diets (Miller et al. 2020). However, the diversity of sequences of other genetically detected taxonomic groups and observations of marine snow objects in gut contents, as well as the trophic position of leptocephali in isotope studies, all suggest that hydrozoans are probably not the main part of the diet of leptocephali (Feunteun et al. 2015; Miller et al. 2013, 2019, 2020).
DNA sequence read composition of the eight most abundant eukaryotic taxonomic groups detected in the gut contents of 35 leptocephali of five anguilliform families (a), and the sequence percent composition of individual leptocephali (horizontal bars) of Anguilla japonica (Aj), A. marmorata (Am), Congridae (CG; Gnathophis, Conger, Bathyuroconger), Eurypharyngidae (EP, Eurypharynx pelecanoides), Muraenidae (MR), and Chlopsidae (CH) (b). Redrawn from Chow et al. (2019)
Laboratory feeding of leptocephali
Finding a diet larvae will eat
After successful artificial maturation and fertilization techniques were found for Japanese eels, the challenge became to find something the larvae would eat (Tanaka 2015). This proved to be quite difficult, because although many things such as zooplankton, formulated micro-diets for marine fish larvae, crustaceans, fish eggs, shrimp, jellyfish, cuttlefish, and the gonads of mussels were offered to first-feeding stage leptos, they would not ingest these materials (Tanaka et al. 2003). They were found to eat rotifers (Tanaka et al. 1995), and later studies confirmed that, but also found that there were some species-specific preferences, with one species, Proales similis, being preferentially ingested (Wullur et al. 2013; Hagiwara et al. 2014; Butts et al. 2016). The larvae were able to digest and assimilate proteins from the rotifers (Kurokawa et al. 1995, 1996), and nutritional enrichment trials were conducted with Proales similis (Tomoda et al. 2014). Tanaka et al. (2001) reported that larvae would also eat boiled egg yolk and a micro-diet, but the larvae, including those that ate rotifers, did not grow or survive. Otake et al. (1995) found that first-feeding leptocephali of pike eels (Muraenesox cinereus) would not eat any of the materials they were offered, which included rotifers, Artemia nauplii, fertilized eggs or larvae of oysters and sea urchins, and several species of phytoplankton.
Then Tanaka et al. (2001) were able to rear Japanese eel larvae for 100 days to reach about 22 mm by feeding them a slurry-type diet that contained shark egg yolk powder (from the spiny dogfish, Squalus acanthias), which is used to increase the nutritional value of food fed to other types of marine fish larvae. The larvae were also fed slurry diets containing other materials such as soybean peptide, vitamins, minerals, or krill extract five times per day, because they would not survive past 30 days by only eating shark egg yolk powder. The negative phototaxis of larvae (Yamada et al. 2009) caused the larvae to swim to the bottom, where they would encounter the slurry and consume it. Most larvae died from starvation between 10 and 15 days after hatching, indicating that many did not eat enough of the slurry to survive the first-feeding stage (Tanaka et al. 2001). The diet was not sufficient for long-term growth, however, because the survival rate at 50 days was ≤ 2%, and only a few survived to 100 days.
There seems to be little published about the larval feeding success of other species of anguillid eel larvae that have been artificially spawned in recent years, but this allowed their eggs and larvae to be observed and studied (Oliveira and Hable 2010; Bouilliart et al. 2015; Butts et al. 2016; Sørensen et al. 2016). The study by Butts et al. (2016) tested various types of food with first-feeding European eel larvae and found that at 16 days after hatching, 23–50% would ingest rotifer paste or the paste with live rotifers or cod roe added. Their feeding behavior was also observed, but the development of diets for the production of large numbers of European eel larvae has not been reported.
Refining artificial diets
The shark egg yolk slurry diet was further refined and the leptocephali survived to the glass eel stage, and the life cycle of the Japanese eel was completed in the laboratory (Tanaka et al. 2003, 2006; Kagawa et al. 2005; Masuda et al. 2011a, 2012; Tanaka 2015). The previous diet was modified to contain krill hydrolysate and the soybean peptide was treated with phytase, and the larvae eating this diet grew to 50–60 mm in total length and began to metamorphose into glass eels at about 250 days after hatching (Tanaka et al. 2003). Other refinements of feeding or temperature regimes achieved metamorphosis in shorter time periods (Masuda et al. 2011a, 2013a). Figure 8 shows what the paste-like food containing shark egg yolk looks like in the intestine of a 48 mm cultured Japanese eel leptocephalus, in comparison to the same larva with an empty gut before feeding. A wide variety of refinements were made to the artificial maturation of the adult eels to obtain higher-quality eggs and to improve the success of rearing the larvae (reviewed by Okamura et al. 2014), but the basic type of diet remained about the same and contained the shark egg powder during that time.
The growth rate of cultured Japanese eel larvae remained lower than that of wild larvae as estimated from their otolith microstructures, and the use of shark eggs for large-scale larval production would not be sustainable, so efforts continued to improve the artificial diet (Okamura et al. 2014; Tanaka 2015). Although various different approaches were tried, an important goal was to replace the dogfish shark egg yolk, and the egg yolk of other species of sharks was also found to be usable (see Masuda et al. 2011b). It was also found that larvae would feed and grow if shark egg powder was replaced with chicken egg yolk, but their growth and survival was lower (Okamura et al. 2013). That study also showed that skinned Antarctic krill was better to add to the diet than krill with their exoskeleton, apparently due to high fluoride levels. The composition of overall protein and specific fatty acids differs between the shark and chicken egg yolk (Okamura et al. 2012a, 2013), so that may be one factor contributing to the difference in effectiveness between the two diet components. It was found that it was useful to add carbohydrates in the form of N-acetylglucosamine, glucose or maltose (see Okamura et al. 2014). Another approach was to replace shark egg with fish protein hydrolysate that had been digested using frozen krill enzymes (Masuda et al. 2013b). Some growth of early larvae was found, but survival rates were even lower than for the chicken egg diet. It was also found that reducing the amount of fat in the artificial diet was beneficial for higher survival and growth for both shark egg and chicken egg diets (Furuita et al. 2014). The optimum dietary level of the highly unsaturated fatty acid, arachidonic acid, for Japanese eel larvae was also recently determined (Shahkar et al. 2016). Another improvement was found by diluting the slurry-type diet with water to make it easier for the larvae to ingest (Yamada et al. 2019; Okamura et al. 2019). Research showed that starting to feed the larvae at 5 days after hatching was advantageous compared to initial feeding at 7 or 8 days (Jinbo et al. 2013).
Food sources in the ocean
Stable isotopic studies on leptocephali
Both before and after the marine snow feeding hypothesis emerged, stable isotopic ratio studies were conducted to evaluate the possible food sources of leptocephali in pelagic food webs. Marine eel leptocephali of Conger myriaster from nearshore waters of Japan had δ15N signatures that were slightly lower than that of POM from the area, and much lower than the levels of other marine animals (Otake et al. 1993). A different study found that leptocephali of that species collected offshore to the south of Japan closer to their spawning area had lower δ15N ratios (Chow et al. 2010), suggesting that the δ15N values of the older larvae had changed to become more similar to the POM in the coastal area. Different isotopic signatures of leptocephali were found on each side of a frontal feature in the western North Pacific (Kimura and Tsukamoto 2006), and then studies compared the signatures of leptocephali with POM collected in the same locations in a wider range of regions (Miyazaki et al. 2011; Feunteun et al. 2015; Liénart et al. 2016; Quattrini et al. 2019; Ghinter et al. 2020). These studies were generally consistent with marine snow being the food source of the leptocephali, but direct correspondence with POM signatures linked with typical fractionation values was usually not seen. This is likely due to several factors, for example, not all of the ingested material being digested or assimilated, that leptocephali likely only feed on specific types of POM (marine snow particles), and that some components of POM such as carbohydrates could be washed out during the POM filtration process (Feunteun et al. 2015; Liénart et al. 2016). Those and other studies analyzed the POM materials obtained from water filtering that retains all particles larger than 0.7 μm. However, that would result in the collection of both free-living photosynthetic and heterotrophic plankton and not just marine snow or other detrital material. Therefore, if leptocephali primarily target marine snow particulate material, then there would be no expectation of observing direct fractionation correspondences with POM, especially if some molecular fraction of the marine snow is soluble in water and is washed through the filters.
Interestingly, though, the isotopic studies also showed that there were taxonomic and geographic differences in the isotopic signatures of leptocephali. Leptocephali that grow to large sizes of about > 150 mm to more than 300 mm (Nemichthyidae and Ariosoma-type of the Congridae; Group 2 in Fig. 9) tended to have lower δ15N ratios than larvae of all the other taxa (including anguillid larvae; Group 1 in Fig. 9) that do not grow larger than about 150 mm (Miyazaki et al. 2011; Feunteun et al. 2015; Liénart et al. 2016; Quattrini et al. 2019; Ghinter et al. 2020). This same general pattern has been found everywhere studies have been conducted, which includes in the North and South Pacific, the western Indian Ocean, and the Gulf of Mexico (Fig. 9). More variability was found in leptocephalus isotopic signatures in the Gulf of Mexico, possibly due to the large input of freshwater from the Mississippi River, but the same pattern of two groups of signatures was seen for most of the larvae (Fig. 9c; Quattrini et al. 2019).

modified from Feunteun et al. 2015), and for seven taxa of leptocephali from over the steep continental slope ~ 250–1000 m (SL, lighter symbols) and the outer slope at ~ 2000–3000 m (OC, darker symbols) in the Gulf of Mexico (redrawn from Quattrini et al. 2019)
Plots of the mean nitrogen (δ15N) and carbon (δ13C) stable isotopic values of various taxa of leptocephali separated into the Group 1 (high δ15N) and Group 2 (low δ13C) categories of Feunteun et al. (2015) for two species of leptocephali in the western North Pacific in relation to particulate organic material (POM) from several depths (a redrawn from Miyazaki et al. 2011), for 12 taxa of leptocephali from west of the Mascarene Plateau in the western Indian Ocean (b
It is unclear what causes the isotopic difference between the two groups of taxa, but consumption of different types of food items or feeding at different depths have been proposed as possible explanations. POM can have different signatures at deeper depths compared to shallow depths (Fig. 9a; Miyazaki et al. 2011), so leptocephali could have different isotopic signatures even if they feed on similar materials at different depths (Ghinter et al. 2020). In the South Pacific, the isotopic signatures of both POM and leptocephali varied with latitude (δ15N values increased from south to north), and showed a geographic linkage between the signatures of the larvae and POM (Liénart et al. 2016; Ghinter et al. 2020). POM signatures also varied with depth in that region, with higher δ15N values being found deeper (200–260 m) than at the surface (5 m) or at 80–145 m within the chlorophyll maximum (Ghinter et al. 2020). Different food sources or physiologies among taxa of leptocephali were also suggested by the differences in fatty acid composition among the leptocephali of three different families in the South Pacific (Fig. 10; Liénart et al. 2016).

(modified from Liénart et al. 2016)
Comparison of the fatty acid composition of Muraenidae (MR), Nemichthys (Nemichthyidae; NM), and Serrivomeridae (SR) separated into polyunsaturated (a PUFA) and saturated (b SFA) fatty acids
Possible food sources
As outlined so far, the studies conducted on leptocephali caught in the ocean have made observations that are consistent with these unusual larvae feeding on marine snow. The objects that have been seen in leptocephalus gut contents, as described above, and the marine organisms detected genetically in gut contents could aggregate into marine snow (Alldredge and Silver 1988), as shown through genetic sequence analysis of marine snow (Ayala et al. 2018; Lundgreen et al. 2019). Terahara et al. (2011) and Chow et al. (2019) also detected sequences of fungi in the gut contents of Japanese eel leptocephali, which would only be ingested with marine snow. In addition, other recent behavioral studies using artificially spawned and reared leptocephali found that the young larvae would ingest phytoplankton exudate or POM type materials (Tomoda et al. 2015; Chow et al. 2017), which is consistent with feeding on marine snow. Basically, no organism has been observed in leptocephalus gut contents that could not have been consumed as part of marine snow aggregates.
However, as discussed below, the easily observable objects in the gut contents may not be the main food source of leptocephali, because marine snow can contain carbohydrates and simple sugars that originate from phytoplankton or bacterial exudates in the form of TEP (Cowen and Holloway 1996; Passow 2002; Engel 2004; Skoog et al. 2008; Mari et al. 2017) that would be an excellent food source for the leptocephali. In addition, higher-magnification imagery of gut contents found that the typical amorphous material also contains small round objects (Miller et al. 2019) that had the characteristics of heterotrophic thraustochytrid protists (Raghukumar 2002; Raghukumar and Damare 2011; Marchan et al. 2018). These single-celled organisms are widely present in both coastal and offshore areas and in marine snow aggregates (Lyons et al. 2005; Li et al. 2013; Bochdansky et al. 2017). For example, marine snow from near Hawaii (Li et al. 2013) and “phytoplankton detritus” aggregates from the North Sea were shown to contain round-shaped thraustochytrid cells (Raghukumar and Shaumann 1993) that closely resemble the possible thraustochytrid aggregates found in the intestines of four different species of leptocephali in the Sargasso Sea (Miller et al. 2019). Images of amorphous materials that flowed out of leptocephalus intestines in the Pacific region also contained many round objects (Fig. 4d–f).
Interestingly, however, Chow et al. (2019) detected DNA sequences of intestinal conoid parasites (phylum Apicomplexa, class Conoidasida, subclass Coccidia and Gregarinasina) in the gut contents of about half (~ 40–60 mm) of the examined leptocephali (~ 18–68 mm) in the western North Pacific (Fig. 7). Those protist parasites have multi-stage life histories and are found in both terrestrial and aquatic animals including marine fishes (Sitjà-Bobadilla and Palenzuela 1996; Molnár et al. 2012; Lovy and Friend 2015; Rosenthal et al. 2016). Some stages such as those found in the fecal material of sea lions (Girard et al. 2016) or aquacultured marine fish (sea bream) intestines (Sitjà-Bobadilla et al. 1996) have round shapes (< 10 μm) that also appear similar to some thraustochytrids. If they are shed in marine animal fecal material, they might be present in marine snow aggregates consumed by leptocephali. Or they could be intestinal parasites on the leptocephali, but there does not seem to be any published information about conoid parasites in fish larvae, so this recent discovery by Chow et al. (2019) needs to be explored in relation to the digestive systems of leptocephali.
Especially for small leptocephali, however, an important question still remains about the range of types of particles they might consume in nature, because small first-feeding leptocephali will eat rotifers or rotifer paste, as mentioned above. The consistent presence of marine snow components in leptocephalus gut contents studies, however, clearly indicates that they feed on marine snow and that directly feeding on individual large organisms does not provide their main food source. The observations that when their normal type of food is absent, young leptocephali will eventually consume things like a paste containing shark egg yolk or squid paste for older larvae (Mochioka et al. 1993), suggest they have some flexibility in what they will consume when faced with starvation in the case of first-feeding larvae. However, extensive feeding trials in aquaculture laboratories have shown that they will not ingest most things, and that a quite specific food source is required for them to grow and reach metamorphosis.
Nutrition, growth, and survival
Although leptocephali may primarily feed on marine snow, they may not digest and assimilate everything they consume, such as fecal pellets or exoskeletal components of dead organisms. The alimentary canal of Japanese eel leptocephali appears to be fully formed and able to absorb food at 7 days after hatching (Ozaki et al. 2006), and its structure remains the same during larval growth (Otake 1996). Leptocephali have proteolytic and other enzymes that are secreted into their intestines (Kurokawa and Pedersen 2003; Pedersen et al. 2003; Kurokawa et al. 2004; Murashita et al. 2013), and they can absorb proteins (Kurokawa et al. 1996) and other materials in their mid- and hind-guts (Otake et al. 1995; Otake 1996). Comparisons of the relative expression levels of different categories of digestive enzymes and nutrient transporter RNA transcripts between wild-caught Japanese eel preleptocephali, leptocephali, and glass eels indicated that leptocephali had high expression levels for proteolytic enzymes and high levels for both amino acid and glucose/fructose transport in the intestinal epithelium (Hsu et al. 2015). But enzymes would likely be unable to quickly digest all components of objects such as fecal pellets, so the assimilation process may be designed to absorb the easily digestible or absorbable components and then continue to ingest more food particles if they are available. Feeding of leptocephali on artificial diets suggests that growth is fastest when they feed on a semi-liquid diet (Yamada et al. 2019; Okamura et al. 2019), so soft easily digested and assimilated materials may be the target of what they consume. For example, when larvacean houses are observed in leptocephalus gut contents, they are usually accompanied by amorphous material, so it is not clear whether the target of the larvae was ingestion of the house or ingestion of other aggregates associated with the house. The houses can be colonized by bacteria and protozoans, however, which could increase their nutritional value (Davoll and Silver 1986). Marine snow and discarded larvacean houses contain a wide variety of bacteria and other microorganisms (Alldredge and Silver 1988; Volkman and Tanoue 2002; Simon et al. 2002; Azam and Malfatti 2007) that could be digested and would contribute to their nutrition. If thraustochytrid cells are present in aggregates that are ingested, they might also be digestible. The thraustochytrid cells are rich in PUFAs and fatty acids (Marchan et al. 2018), which are present in leptocephali (Fig. 10a; Deibel et al. 2012; Liénart et al. 2016), and POM has been found to contain various lipid compounds (Alldredge 1979; Wakeham and Lee 1989). Thraustochytrids have been found to be suitable for replacing significant portions of fish meal and fish oil in diets of young fish reared in aquaculture (Perez-Velazquez et al. 2018).
Another likely nutritional source within marine snow are the carbohydrate-containing TEP molecules that can facilitate aggregation of marine snow particles (see Mari et al. 2017). Chemical analyses of the contents of POM and DOC in the ocean have provided information about the likely prevalence of carbohydrates in the ocean (Skoog et al. 2008; Engel and Händel 2011; Engel et al. 2012) and their likely importance in the aggregation of marine snow (Alldredge 2001; Engel et al. 2004). Marine snow can contain various carbohydrates in the form of polysaccharides and simple sugars such as glucose and galactose (Cowen and Holloway 1996; Skoog et al. 2008). These sugars are the building-blocks of GAG molecules (Mende et al. 2016), so they should be easily assimilated and converted into the GAG compounds (predominantly hyaluronan) that are used for energy storage and for conversion into new tissues during metamorphosis (Pfeiler et al. 2002; Kawakami et al. 2009, 2014; Okamura et al. 2018).
Therefore, the amount of TEP released in each area could have a major effect on the amount and quality of marine snow available for leptocephali to consume. TEP was present throughout the upper 300 m in the Japanese eel spawning area, with the highest concentrations being present in the upper 125 m above the chlorophyll maximum, which is similar to other offshore oligotrophic areas (Kodama et al. 2014). TEP was also most abundant in the upper 100 m in the northern Sargasso Sea along with protein-containing particles (Cisternas-Novoa et al. 2015). Eukaryotic phytoplankton-exudate TEP was observed to be ingested by 10–20 day-old artificially spawned leptocephali in the laboratory (Tomoda et al. 2015), so these types of exudate materials may be important in the nutrition of leptocephali, especially after phytoplankton blooms when concentrations would be highest. In contrast, it remains unclear how much TEP is produced by the highly abundant cyanobacteria Prochlorococcus except when they die (Iuculano et al. 2017). That type of cyanobacteria (Partensky et al. 1999) appears to alternate in abundance (high during low-nutrient conditions) with eukaryotic phytoplankton (abundant during high-nutrient conditions) in oligotrophic waters (see Miller et al. 2016). In addition, because TEP is lighter than water, its presence can likely slow down the sinking of particulate materials that contain it (Mari et al. 2017), which could provide secondary advantages for particle availability for the feeding and growth of leptocephali during high-TEP conditions.
The growth rates of leptocephali have been studied in a few species using otolith microstructures, but it is not yet known whether the rates can vary based on the types of food materials that are available for the larvae to feed on. Anguillid larvae seem to grow at rates of about 0.3–0.6 mm day−1, with faster rates occurring in tropical species compared to temperate ones (Kuroki et al. 2014), and most marine eel larvae grow at rates of about 0.4–0.7 mm day−1 (Miller 2009). However, near the continental shelf in the eastern Gulf of Mexico, leptocephali were suggested to have much higher and more variable growth rates (> 1 mm day−1; Bishop et al. 2000).
The availability or quality of marine snow has been hypothesized to have the potential to influence the first-feeding early larval survival of leptocephali, such as in the Sargasso Sea where several species of anguillid and mesopelagic eels spawn in the same areas during the same season (Miller et al. 2016). With many millions of eggs being spawned by each anguillid female, if there is not enough food available for the recently hatched larvae, they could die of starvation due to competition with other larvae. This was proposed as a possible mechanism that could link ocean–atmosphere changes affecting marine snow production and recruitment fluctuations caused by variations in early larval survival (Miller et al. 2016). After the larvae grow larger, however, they are likely quite resistant to starvation due to the large amount of GAG energy storage compounds in their body. In fact, laboratory-reared Japanese eel larvae were observed to be able to swim continuously for about 2 months with no food (Miller and Tsukamoto 2017), which illustrated the resistance of large larvae to starvation. Interestingly, once a size threshold of about ~ 40 mm is reached, a lack of food triggers the onset of metamorphosis into the glass eel stage in Japanese eel larvae (Okamura et al. 2012b).
Feeding ecology of leptocephali
Information has accumulated that suggests leptocephali have a feeding strategy based on consuming particles that can be categorized as marine snow, but small leptocephali are also capable of consuming soft-bodied organisms if they have the opportunity, based on laboratory observations. However, the observations reviewed here can only be explained by the studied leptocephali having consumed marine snow aggregates, even if some types of small living organisms are directly ingested. The DNA sequence studies on European eel larvae gut contents (Riemann et al. 2011; Ayala et al. 2018) and those of Pacific species of leptocephali (Chow et al. 2019) contain a wide diversity of taxa that are consistent with the majority of the taxa being ingested as part of marine snow. This general pattern seems to fit available information, but the isotopic signature studies indicated that differences exist in some aspects of the feeding ecology or physiology of some families of leptocephali (Feunteun et al. 2015; Liénart et al. 2016), which are not yet understood.
Another factor to consider when trying to understand the feeding ecology of leptocephali is the morphology and size of their heads and jaws, which can vary widely among taxa and the size of larvae (Figs. 1 and 2). The patterns of size-scaling of the length and number of teeth in leptocephali suggest that they can feed on overlapping sizes of particles as they grow larger. The relative size and number of teeth change markedly as the leptocephali grow larger (Castle 1963, 1965, 1970; Miller et al. 2019), which may be related to the different sizes of larvae feeding on overlapping sizes of food particles (Miller et al. 2019). Using the leptocephali of the mesopelagic eel Derichthys serpentinus (Castle 1970) that have a similar body shape and size range as most anguillid leptocephali as an example, the possible size of food particles can be compared to the teeth and mouth sizes of small, medium, and large larvae (Fig. 11). This suggests that the appropriate sizes of particles for first-feeding larvae would be about 0.25 mm or less in diameter, 0.5 mm particles seem appropriate for 18 mm larvae, and particles both smaller or larger than 1 mm could be consumed by 37 mm larvae (Fig. 11). Considering that most species of leptocephali reach sizes of 60–150 mm (Ariosoma and Nemichthyidae can reach 300 mm or larger), the size of particles could be several millimeters bigger. The presence of larger numbers of more closely spaced teeth in the larger larvae may be an adaptation to able to retain smaller particles in the mouth before swallowing them. In contrast, the large forward-pointing teeth of the small larvae seem to be appropriate for grasping large soft particles (Westerberg 1990). This suggests that there can be considerable overlap in the sizes of particles consumed by the wide size range of larvae. This may be an important adaptation because small marine snow particles (0.05–0.53 mm) were found to be much more abundant (15–23 particles L−1) than medium (0.53–1.06 mm) and large (1.06–4.0 mm) particles (< 0.23 particles L−1) in the upper 400 m of the Sargasso Sea (Munk et al. 2018).

modified from Castle (1970)
Comparison of the relative sizes of the teeth and jaws of three sizes of Derichthys serpentinus leptocephali and three sizes of food particles (i.e., marine snow particles), all at the same size scale. As the larvae grow in size, more teeth are added, making the gaps between the teeth remain similar, which seemingly allows the larger larvae to retain the smaller food particles appropriate for the smallest larvae as well as larger ones. The leptocephalus head images were
Unfortunately, marine snow particles in the lower size ranges < 1 mm that are likely to be consumed by medium to small leptocephali are likely to be fragile and difficult to collect for studying them. Most detailed studies on marine snow seem to have been conducted on large particles in high-productivity regions (e.g., Lampitt et al. 1993; Cowen and Holloway 1996) or large particles in the oligotrophic Sargasso Sea (Ayala et al. 2018; Lundgreen et al. 2019) that were likely larger than early-stage leptocephali can eat (Miller et al. 2020). Therefore, it appears that there is a gap in knowledge about the smaller particles that leptocephali feed on in offshore areas. It is also likely that the types of these small particles can be highly variable in their content and abundance spatially and temporally, so what is consumed would depend on the biological activity within the food web of each area at each time of sampling. Leptocephali are present from over the continental shelf in high-productivity shallow waters, to far out into the oligotrophic subtropical gyres (Miller and Tsukamoto 2020), so the food material could vary widely within the quite general category of marine snow particulates.
Future perspectives
This review indicates that leptocephali appear to feed on some types of marine snow in the ocean, which can contain a wide range of materials including carbohydrates, but they will feed and grow on other materials in the laboratory. More studies are needed on each taxonomic group of leptocephali and the detailed characteristics of marine snow in many different regions to gain a better understanding of which larvae are eating what types of marine snow particles and what nutrition they might get from consuming them. Future research should combine a variety of techniques that include simultaneous microscopy and DNA sequence analyses of gut contents and marine snow particles. This new information might also help guide further refinements of the diets fed to artificially cultured leptocephali, regarding nutritional requirements, what types of materials or chemical compounds the larvae may be attracted to, and whether there might be alternative methods for presenting the food to the larvae. At present, the paste-type of food fed to larvae in the aquaculture setting does not appear to be directly related to the feeding ecology of wild larvae, but instead seems to consists of materials that the larvae will learn to eat to stay alive as an alternative to starvation. They appear to be attracted to the paste because of chemical compounds related to the egg yolk of sharks or chickens. This type of paste diet can be successful for rearing of larvae until metamorphosis, at least for Japanese eel larvae, but the addition of the paste into the tanks and then its removal to maintain water quality creates a logistical challenge to efficient, lower-cost, mass production of glass eels. Efforts are ongoing to address this challenge, which if successful can produce glass eels to help reduce the impact of harvesting wild glass eel seedlings for aquaculture.
References
Alldredge AL (1976) Discarded appendicularian houses as sources of food, surface habitats, and particulate organic matter in planktonic environments. Limnol Oceanogr 21:14–23
Alldredge AL (1979) The chemical composition of macroscopic aggregates in two neretic seas. Limnol Oceanogr 24:855–866
Alldredge AL (2000) Interstitial dissolved organic carbon (DOC) concentrations within sinking marine aggregates and their potential contribution to carbon flux. Limnol Oceanogr 45:1245–1253
Alldredge AL (2001) Particle aggregation dynamics. In: Encyclopedia of ocean sciences, pp 2090–2097
Alldredge AL, Silver MW (1988) Characteristics, dynamics and significance of marine snow. Progr Oceanogr 20:41–82
Alldredge AL, Passow U, Logan BE (1993) The abundance and significance of a class of large, transparent organic particles in the ocean. Deep-Sea Res I 40:1131–1140
Ayala DJ, Munk P, Lundgreen RBC, Traving SJ, Jaspers C, Jørgensen TS, Hansen LH, Riemann L (2018) Gelatinous plankton is important in the diet of European eel (Anguilla anguilla) larvae in the Sargasso Sea. Sci Rep 8:6156
Azam F, Malfatti F (2007) Microbial structuring of marine ecosystems. Nat Rev Microbiol 5:782–791
Bishop RE, Torres JJ (1999) Leptocephalus energetics: metabolism and excretion. J Exp Biol 202:2485–2493
Bishop RE, Torres JJ, Crabtree RE (2000) Chemical composition and growth indices in leptocephalus larvae. Mar Biol 137:205–214
Bochdansky AB, Clouse MA, Herndl GJ (2017) Eukaryotic microbes, principally fungi and labyrinthulomycetes, dominate biomass on bathypelagic marine snow. ISME J 11:362–373
Bouilliart M, Tomkiewicz J, Lauesen P, De Kegel B, Adriaens D (2015) Musculoskeletal anatomy and feeding performance of prefeeding engyodontic larvae of the European eel (Anguilla anguilla). J Anat 227:325–340
Butts IAE, Sørensen SR, Politis SN, Tomkiewicz J (2016) First feeding by European eel larvae: a step towards closing the life cycle in captivity. Aquaculture 464:451–458
Castle PHJ (1963) The systematics, development and distribution of two eels of the genus Gnathophis (Congridae) in Australasian waters. Zool Publ Vict Univ Well 34:1–47
Castle PHJ (1965) Leptocephali of the Nemichthyidae, Serrivomeridae, Synaphobranchidae and Nettastomatidae in Australasian waters. Trans Roy Soc NZ 5:131–146
Castle PHJ (1970) Distribution, larval growth, and metamorphosis of the eel Derichthys serpentinus Gill, 1884 (Pisces, Derichthyidae). Copeia 1970:444–452
Castonguay M, McCleave JD (1987) Vertical distributions, diel and ontogenetic vertical migrations and net avoidance of leptocephali of Anguilla and other common species in the Sargasso Sea. J Plankt Res 9:195–214
Chikaraishi Y, Ogawa NO, Kashiyama Y, Takano Y, Suga H, Tomitani A, Miyashita H, Kitazato H, Ohkouchi N (2009) Determination of aquatic foodweb structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol Oceanogr Methods 7:740–750
Chow S, Kurogi H, Katayama S, Ambe D, Okazaki M, Watanabe T, Ichikawa T, Kodama M, Aoyama J, Shinod A, Watanabe S, Tsukamoto K, Miyazaki S, Kimura S, Yamada Y, Nomura K, Tanaka H, Kazeto Y, Hata K, Handa T, Tawa A, Mochioka N (2010) Japanese eel Anguilla japonica do not assimilate nutrition during the oceanic spawning migration: evidence from stable isotope analysis. Mar Ecol Prog Ser 402:233–238
Chow S, Kurogi H, Watanabe S, Matsunari H, Sudo R, Nomura K, Tanaka H, Furuita H, Nishimoto A, Higuchi TJ, Tomoda T (2017) Onboard rearing attempts for the Japanese eel leptocephali using POM-enriched water collected in the western North Pacific. Aquat Living Resour 30:38
Chow S, Inaba N, Nagai S, Kurogi H, Nakamura Y, Yanagimoto T, Tanaka H, Hasegawa D, Asakura T, Kikuchi J, Tomoda T, Kodama T (2019) Molecular diet analysis of Anguilliformes leptocephalus larvae collected in the western North Pacific. PLoS ONE 14(11):e0225610
Cisternas-Novoa C, Lee C, Engel A (2015) Transparent exopolymer particles (TEP) and Coomassie stainable particles (CSP): Differences between their origin and vertical distributions in the ocean. Mar Chem 175:56–71
Cowen JP, Holloway CF (1996) Structural and chemical analysis of marine aggregates: in situ macrophotography and laser confocal and electron microscopy. Mar Biol 126:163–174
Davoll PJ, Silver MW (1986) Marine snow aggregates: life history sequence and microbial community of abandoned larvacean houses from Monterey Bay, California. Mar Ecol Prog Ser 33:111–120
Deibel D, Parrish CC, Grønkjaer P, Munk P, Nielsen TG (2012) Lipid class and fatty acid content of the leptocephalus larva of tropical eels. Lipids 47:623–634
Donnelly J, Torres JJ, Crabtree RE (1995) Proximate composition and nucleic acid content of premetamorphic leptocephalus larvae of the congrid eel Ariosoma balearicum. Mar Biol 123:851–858
Engel A (2004) Distribution of transparent exoploymer particles (TEP) in the northeast Atlantic Ocean and their potential significance for aggregation processes. Deep-Sea Res I 51:83–92
Engel A, Händel N (2011) A novel protocol for determining the concentration and composition of sugars in particulate and in high molecular weight dissolved organic matter (HMW-DOM) in seawater. Mar Chem 127:180–191
Engel A, Thoms S, Riebesell U, Rochelle-Newall E, Zondervan I (2004) Polysaccharide aggregation as a potential sink of marine dissolved organic carbon. Nature 428:929–932
Engel A, Harlay J, Piontek J, Chou L (2012) Contribution of combined carbohydrates to dissolved and particulate organic carbon after the spring bloom in the northern Bay of Biscay (North-Eastern Atlantic Ocean). Cont Shelf Res 45:42–53
Feunteun E, Miller MJ, Carpentier A, Aoyama J, Dupuy C, Kuroki M, Pagano M, Réveillac E, Sellos D, Watanabe S, Tsukamoto K, Otake T (2015) Stable isotopic composition of anguilliform leptocephali and other food web components from west of the Mascarene Plateau. Progr Oceanogr 137:69–83
Furuita H, Murashita K, Matsunari H, Yamamoto T, Nagao J, Nomura K, Tanaka H (2014) Decreasing dietary lipids improves larval survival and growth of Japanese eel Anguilla japonica. Fish Sci 80:581–587
Ghinter L, Dupuy C, Miller MJ, Carpentier A, Lefrançois C, Acou A, Aoyama J, Kuroki M, Liénart C, Watanabe S, Tsukamoto K, Otake T, Feunteun E (2020) Microbial functional structure and stable isotopic variation of leptocephali across three current zones in the western South Pacific. Prog Oceanogr 182:102264
Girard YA, Johnson CK, Fritz HM, Shapiro K, Packham AE, Melli AC, Carlson-Bremer D, Gulland FM, Rejmanek AE, Conrad PA (2016) Detection and characterization of diverse coccidian protozoa shed by California sea lions. Int J Parasitol Parasites Wildl 5:5–16
Govoni J (2010) Feeding on protists and particulates by the leptocephali of the worm eels Myrophis spp. (Teleostei, Anguilliformes, Ophichthidae), and the potential energy contribution of large aloricate protozoa. Scientia Marina 74:339–344
Hagiwara A, Wullur S, Marcial HS, Hirai N, Sakakura Y (2014) Euryhaline rotifer Proales similis as initial live food for rearing fish with small mouth. Aquaculture 432:470–474
Hebel DV, Karl DM (2001) Seasonal, interannual and decadal variations in particulate matter concentrations and composition in the subtropical North Pacific Ocean. Deep-Sea Res II 48:1669–1695
Hsu HY, Chen SH, Cha YR, Tsukamoto K, Lin CY, Han YS (2015) De novo assembly of the whole transcriptome of the wild embryo, preleptocephalus, leptocephalus, and glass eel of Anguilla japonica and deciphering the digestive and absorptive capacities during early development. PLoS ONE 10:e0139105
Hulet WC (1978) Structure and functional development of the eel leptocephalus Ariosoma balearicum (De La Roche, 1809). Phil Trans Royal Soc Lond Biol Sci 282:107–138
Hulet WH, Robins CR (1989) The evolutionary significance of the leptocephalus larva. In: Böhlke EB (ed) Leptocephali. Fishes of the Western North Atlantic. Part 9, vol 2. Sears Foundation for Marine Research, New Haven, pp 669–677
Hunter JR (1981) Feeding ecology and predation of marine fish larvae. In: Lasker R (ed) Marine fish larvae: morphology, ecology and relation to fisheries. Washington Sea Grant Program, Seattle, pp 33–77
Iuculano F, Mazuecos IP, Reche I, Agusti S (2017) Prochlorococcus as a possible source for transparent exopolymer particles (TEP). Front Microbiol 8:709
Jinbo T, Masuda Y, Imaizumi H, Hashimoto H, Matsuda K, Nagao J, Tanaka H (2013) Effects of the timing of initial feeding on growth and survival of Japanese eel Anguilla japonica larvae. Aquaculture Sci 61:403–406 (in Japanese with English abstract)
Kagawa H, Tanaka H, Ohta H, Unuma T, Nomura K (2005) The first success of glass eel production in the world: basic biology on fish reproduction advances new applied technology in aquaculture. Fish Physiol Biochem 31:193–199
Kawakami Y, Oku H, Nomura K, Gorie S, Ohta H (2009) Metabolism of a glycosaminoglycan during metamorphosis in the Japanese conger eel, Conger myriaster. Res Lett Biochem 2009(4):251731. https://doi.org/10.1155/2009/251731
Kawakami Y, Nomura K, Tanaka H (2014) Growth promoting effect of hyaluronan synthesis promoting substances on Japanese eel leptocephali. PLoS ONE 9(6):e98688
Kimura S, Tsukamoto K (2006) The salinity front in the North Equatorial Current: a landmark for the spawning migration of the Japanese eel (Anguilla japonica) related to the stock recruitment. Deep-Sea Res II 53:315–325
Kiørboe T (2000) Colonization of marine snow aggregates by invertebrate zooplankton: abundance, scaling, and possible role. Limnol Oceanogr 45:479–484
Kodama T, Kurogi H, Okazaki M, Jinbo T, Chow S, Tomoda T, Ichikawa T, Watanabe T (2014) Vertical distribution of transparent exopolymer particle (TEP) concentration in the oligotrophic western tropical North Pacific. Mar Ecol Prog Ser 513:29–37
Kurokawa T, Pedersen BH (2003) The digestive system of eel larvae. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer-Verlag, Tokyo, pp 435–444
Kurokawa T, Kagawa H, Ohta H, Tanaka H, Okuzawa K, Hirose K (1995) Development of digestive organs and feeding ability in larvae of Japanese eel (Anguilla japonica). Can J Fish Aquat Sci 52:1030–1036
Kurokawa T, Tanaka H, Kagawa H, Ohta H (1996) Absorption of protein molecules by the rectal cells in eel larvae Anguilla japonica. Fish Sci 62:832–833
Kurokawa T, Iinuma N, Unuma T, Tanaka H, Kagawa H, Ohta H, Suzuki T (2004) Development of endocrine system regulating exocrine pancreas and estimation of feeding and digestive ability in Japanese eel larvae. Aquaculture 234:513–525
Kurokawa T, Koshio M, Kaiya H, Hashimoto H, Nomura K, Uji S, Awaji M, Gen K, Tanaka H (2011) Distribution of pepsinogen- and ghrelin-producing cells in the digestive tract of Japanese eel (Anguilla japonica) during metamorphosis and the adult stage. Gen Comp Endocrinol 173:475–482
Kuroki M, Miller MJ, Tsukamoto K (2014) Diversity of early life history traits in freshwater eels and the evolution of their oceanic migrations. Can J Zool 92:749–770
Lampitt RS, Wishner KF, Turley CM, Angel MV (1993) Marine snow studies in the northeast Atlantic Ocean: distribution, composition and role as a food source for migrating plankton. Mar Biol 116:689–702
Li Q, Wang X, Liu X, Jiao N, Wang G (2013) Abundance and novel lineages of thraustochytrids in Hawaiian waters. Microb Ecol 66:823–830
Liénart C, Feunteun E, Miller MJ, Aoyama J, Mortillaro J-M, Hubas C, Kuroki M, Watanabe S, Dupuy C, Carpentier A, Otake T, Tsukamoto K, Meziane T (2016) Geographic analyses of stable isotopic and fatty acid composition of three families of anguilliform leptocephali in the western South Pacific. Mar Ecol Prog Ser 544:225–241
Lovy J, Friend SE (2015) Intestinal coccidiosis of anadromous and landlocked alewives, Alosa pseudoharengus, caused by Goussia ameliae n. sp. and G. alosii n. sp. (Apicomplexa: Eimeriidae). Int J Parasitol Parasites Wildl 4:159–170
Lundgreen RBC, Jaspers C, Traving S, Ayala DJ, Lombard F, Grossart H-S, Nielsen TG, Munk P, Riemann L (2019) Eukaryotic and cyanobacterial communities associated with marine snow particles in the oligotrophic Sargasso Sea. Sci Rep 8:8891
Lüskow F, Neitzel P, Miller MJ, Jaspers C, Marohn L, Wysujack K, Freese M, Pohlmann JD, Hanel R (2019) Distribution and abundance of net-captured calycophoran siphonophores and other gelatinous zooplankton in the Sargasso Sea European eel spawning area. Mar Biodiv 49:2333–2349
Lyons MM, Ward JE, Smolowitz R, Uhlinger KR, Gast RJ (2005) Lethal marine snow: pathogen of bivalve mollusc concealed in marine aggregates. Limnol Oceanogr 50:1983–1988
Mapstone GM (2014) Global diversity and review of siphonophorae (Cnidaria: Hydrozoa). PLoS ONE 9(2):e87737
Marchan LF, Lee Chang KJ, Nichols PD, Mitchell WJ, Polglase JL, Gutierreza T (2018) Taxonomy, ecology and biotechnological applications of thraustochytrids: a review. Biotechnol Adv 36:26–46
Mari X, Passow U, Migon C, Burd AB, Legendre L (2017) Transparent exopolymer particles: effects on carbon cycling in the ocean. Prog Oceanogr 151:13–37
Masuda Y, Imaizumi H, Oda K, Hashimoto H, Teruya K, Usuki H (2011) Japanese eel Anguilla japonica larvae can metamorphose into glass eel within 131 days after hatching in captivity. Nippon Suisan Gakkaishi 77:416–418 (in Japanese with English abstract)
Masuda Y, Imaizumi H, Hashimoto H, Oda K, Furuita H, Matsunari H, Teruya K, Usuki H (2011) Eggs of the tiger shark Galeocerdo cuvier or gulper shark Centrophorus atromarginatus as food for early-stage larvae of the Japanese eel Anguilla japonica. J Fish Technol 4:7–13 (in Japanese with English abstract)
Masuda Y, Imaizumi H, Oda K, Hashimoto H, Usuki H, Teruya K (2012) Artificial completion of the Japanese eel, Anguilla japonica, life cycle: challenge to mass production. Bull Fish Res Agency 35:111–117
Masuda Y, Jinbo T, Imaizumi H, Hashimoto H, Oda K, Matsuda K, Teruya K, Usuki H (2013) Regulation of water temperature, feeding frequency and larval stocking density leads to shorten the duration of larval stage of Japanese eel, Anguilla japonica. Nippon Suisan Gakkaishi 79:198–205 (in Japanese with English abstract)
Masuda Y, Jinbo T, Imaizumi H, Furuita H, Matsunari H, Murashita K, Jujimoto H, Nagao J, Kawakami Y (2013) A step forward in development of fish protein hydrolysate-based diets for larvae of Japanese eel Anguilla japonica. Fish Sci 79:681–688
Mende M, Bednarek C, Wawryszyn M, Sauter BMB, Schepers U, Bräse S (2016) Chemical synthesis of glycosaminoglycans. Chem Rev 116:8193–8255
Miller MJ (2009) Ecology of anguilliform leptocephali: remarkable transparent fish larvae of the ocean surface layer. Aqua-BioScience Monogr 2:1–94
Miller MJ, Tsukamoto K (2004) An introduction to leptocephali: Biology and identification. Ocean Research Institute, University of Tokyo
Miller MJ, Tsukamoto K (2017) The ecology of oceanic dispersal and survival of anguillid leptocephali. Can J Fish Aquat Sci 74:958–971
Miller MJ, Tsukamoto K (2020) The behavioral ecology and distribution of leptocephali: marine fish larvae with unforeseen abilities. Mar Biol 167:168
Miller MJ, Nakamura Y, Shibuno T, Tsukamoto K (2010) Leptocephali collected in light traps near coral reef habitats of Ishigaki Island in the southern Ryukyu Island chain. Coast Mar Sci 34:47–54
Miller MJ, Otake T, Aoyama J, Wouthuyzen S, Suharti S, Sugeha HY, Tsukamoto K (2011) Observations of gut contents of leptocephali in the North Equatorial Current and Tomini Bay, Indonesia. Coastal Mar Sci 35:277–288
Miller MJ, Chikaraishi Y, Ogawa NO, Yamada Y, Tsukamoto K, Ohkouchi N (2013) A low trophic position of Japanese eel larvae indicates feeding on marine snow. Biol Lett 9:20120826
Miller MJ, Feunteun E, Tsukamoto K (2016) Did a “perfect storm” of oceanic changes and continental anthropogenic impacts cause Northern Hemisphere anguillid recruitment reductions? ICES J Mar Sci 73:43–56
Miller MJ, Marohn L, Wysujack K, Freese M, Pohlmann J-D, Westerberg H, Tsukamoto K, Hanel R (2019) Morphology and gut contents of anguillid and marine eel larvae in the Sargasso Sea. Zoologischer Anzeiger 279:138–151
Miller MJ, Hanel R, Feunteun E, Tsukamoto K (2020) The food source of Sargasso Sea leptocephali. Mar Biol 167:57
Miyazaki S, Kim H-Y, Zenimoto K, Kitagawa T, Miller MJ, Kimura S (2011) Stable isotope analysis of two species of anguilliform leptocephali (Anguilla japonica and Ariosoma major) relative to their feeding depth in the North Equatorial Current region. Mar Biol 158:2555–2564
Mochioka N, Iwamizu M (1996) Diet of anguillid larvae: leptocephali feed selectively on larvacean houses and fecal pellets. Mar Biol 125:447–452
Mochioka N, Mochioka N, Iwamizu M, Kanda T (1993) Leptocephalus eel larvae will feed in aquaria. Environ Biol Fish 36:381–384
Molnár K, Ostoros G, Nunams-Morel D, Rosenthal BM (2012) Eimeria that infect fish are diverse and are related to, but distinct from, those that infect terrestrial vertebrates. Infect Genet Evol 12:1810–1815
Munk P, Nielsen TG, Jaspers C, Ayala DJ, Tang KW, Lombard F, Riemann L (2018) Vertical structure of plankton communities in areas of European eel larvae distribution in the Sargasso Sea. J Plankton Res 40:362–375
Murashita K, Furuita H, Matsunari H, Yamamoto T, Awaji M, Nomura K, Nagao J, Tanaka H (2013) Partial characterization and ontogenetic development of pancreatic digestive enzymes in Japanese eel Anguilla japonica larvae. Fish Physiol Biochem 39:895–905
Okamura A, Yamada Y, Horie N, Mikawa N, Tanaka S, Kobayashi H, Tsukamoto K (2012) Hen egg yolk and skinned krill as possible foods for rearing leptocephalus larvae of Anguilla japonica Temminck and Schlegel. Aquac Res 44:1531–1538
Okamura A, Yamada Y, Mikawa N, Horie N, Tsukamoto K (2012) Effect of starvation, body size and temperature on the onset of metamorphosis in Japanese eel (Anguilla japonica). Can J Zool 90:1378–1385
Okamura A, Yamada Y, Horie N, Mikawa N, Tanaka S, Kobayashi H, Tsukamoto K (2013) Hen egg yolk and skinned krill as possible foods for rearing leptocephalus larvae of Anguilla japonica Temminck & Schlegel. Aquacult Res 44:1531–1538
Okamura A, Horie N, Mikawa N, Yamada Y, Tsukamoto K (2014) Recent advances in artificial production of glass eels for conservation of anguillid eel populations. Ecol Freshw Fish 23:95–110
Okamura A, Sakamoto Y, Yamada Y, Tsukamoto K (2018) Accumulation of hyaluronan in reared Japanese eel Anguilla japonica during early ontogeny. Aquaculture 497:220–225
Okamura A, Y Yamada, N Horie, N Mikawa, and K Tsukamoto (2019) Long-term rearing of Japanese eel larvae using a liquid-type diet: food intake, survival and growth. Fish Sci 85:687–694
Oliveira K, Hable WE (2010) Artificial maturation, fertilization, and early development of the American eel (Anguilla rostrata). Can J Zool 88:1121–1128
Otake T (1996) Fine structure and function of the alimentary canal in leptocephali of the Japanese eel Anguilla japonica. Fish Sci 62:28–34
Otake T, Nogami K, Maruyama K (1993) Dissolved and particulate organic matter as possible food sources for eel leptocephali. Mar Ecol Prog Ser 92:27–34
Otake T, Hirokawa J, Fujimoto H, Imaizumi K (1995) Fine structure and function of the gut epithelium of pike eel larvae. J Fish Biol 47:126–142
Ozaki Y, Tanaka H, Kagawa H, Ohta H, Adachi S, Yamauchi K (2006) Fine structure and differentiation of the alimentary canal in captive-bred Japanese eel Anguilla japonica preleptocephali. Fish Sci 72:13–19
Partensky F, Hess WR, Vaulot D (1999) Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol Molec Biol Rev 63:106–127
Passow U (2002) Transparent exopolymer particles (TEP) in aquatic environments. Progr Oceanogr 55:287–333
Pedersen BH, Ueberscha B, Kurokawa T (2003) Digestive response and rates of growth in pre-leptocephalus larvae of the Japanese eel Anguilla japonica reared on artificial diets. Aquaculture 215:321–338
Perez-Velazquez M, Gatlin DM III, González-Félix ML, García-Ortega A (2018) Partial replacement of fishmeal and fish oil by algal meals in diets of red drum Sciaenops ocellatus. Aquaculture 487:41–50
Pfeiler E (1986) Towards an explanation of the developmental strategy in leptocephalus larvae of marine fishes. Environ Biol Fish 15:3–13
Pfeiler E (1999) Developmental physiology of elopomorph leptocephali. Comp Biochem Physiol A 123:113–128
Pfeiler E, Govoni JJ (1993) Metabolic rates in early life history stages of elopomorph fishes. Biol Bull 185:277–283
Pfeiler E, Toyoda H, Williams MD, Nieman RA (2002) Identification, structural analysis and function of hyaluronan in developing fish larvae (leptocephali). Comp Biochem Physiol B 132:443–451
Pilskaln CH, Villareal TA, Dennett M, Darkangelo-Wood C, Meadows G (2005) High concentrations of marine snow and diatom algal mats in the North Pacific Subtropical Gyre: implications for carbon and nitrogen cycles in the oligotrophic ocean. Deep-Sea Res I 52:2315–2332
Quattrini AM, McClain-Counts J, Artabane SJ, Roa-Varon A, McIver TC, Rhode M, Ross SW (2019) Assemblage structure, vertical distributions, and stable isotopic compositions of anguilliform leptocephali in the Gulf of Mexico. J Fish Biol https://doi.org/10.1111/jfb.13933
Raghukumar S (2002) Ecology of the marine protists, the Labyrinthulomycetes (thraustochytrids and labyrinthulids). Eur J Protistol 38:127–145
Raghukumar S, Shaumann K (1993) An epifluorescence microscopy method for direct detection and enumeration of the thraustochytrids. Limnol Oceanogr 38:182–187
Raghukumar S, Damare VS (2011) Increasing evidence for the important role of Labyrinthulomycetes in marine ecosystems. Bot Mar 54:3–11
Riemann L, Alfredsson H, Hansen MM, Als TD, Nielsen TG, Munk P, Aarestrup K, Maes GE, Sparholt H, Petersen MI, Bachler M, Castonguay M (2010) Qualitative assessment of the diet of European eel larvae in the Sargasso Sea resolved by DNA barcoding. Biol Lett 6:819–822
Rosenthal BM, Dunams-Morel OG, Molnár K (2016) Coccidian parasites of fish encompass profound phylogenetic diversity and gave rise to each of the major parasitic groups in terrestrial vertebrates. Infect Genet Evol 40:219–227
Sato R, Tanaka Y, Ishimaru T (2003) Species-specific house productivity of appendicularians. Mar Ecol Prog Ser 259:163–172
Skoog A, Alldredge A, Passow U, Dunne J, Murray J (2008) Neutral aldoses as source indicators for marine snow. Mar Chem 108:195–206
Shahkar E, Yun H, Lee S, Kim D-J, Kim S-K, Lee BI, Bai SC (2016) Evaluation of the optimum dietary arachidonic acid level and its essentiality based on growth and non-specific immune responses in Japanese eel, Anguilla japonica. Aquaculture 452:209–216
Shanks AL, Walters K (1997) Holoplankton, meroplankton, and meiofauna associated with marine snow. Mar Ecol Progr Ser 156:75–86
Simon M, Grossart H, Schweitzer B, Ploug H (2002) Microbial ecology of organic aggregates in aquatic ecosystems. Aquat Microb Ecol 28:175–211
Sitjà-Bobadilla A, Palenzuela O (1996) Light microscopic description of Eimeria sparis sp. nov. and Goussia sparis sp. nov. (Protozoa: Apicomplexa) from Sparus aurata L. (Pisces: Teleostei). Parasitol Res 82:323–332
Smith DG (1989) Introduction to leptocephali. In: Böhlke, EB (ed) Fishes of the Western North Atlantic. Part 9, Vol 2. Sears Found Mar Res, New Haven, pp 657–668
Sørensen SR, Tomkiewicz J, Munk P, Butts IAE, Nielsen A, Lauesen P, Graver C (2016) Ontogeny and growth of early life stages of captive-bred European eel. Aquaculture 456:50–61
Taguchi S (1982) Seasonal study of fecal pellets and discarded houses of appendicularia in a subtropical inlet, Kaneohe Bay, Hawaii. Estuar Coast Shelf Sci 14:545–555
Tanaka H (2015) Progression in artificial seedling production of Japanese eel Anguilla japonica. Fish Sci 81:11–19
Tanaka H, Kagawa H, Ohta H, Okuzawa K, Hirose K (1995) The first report of eel larvae ingesting rotifers. Fish Sci 61:171–172
Tanaka H, Kagawa H, Ohta H (2001) Production of leptocephali of Japanese eel (Anguilla japonica) in captivity. Aquaculture 201:51–60
Tanaka H, Kagawa H, Ohta H, Unuma T, Nomura K (2003) The first production of glass eel in captivity: fish reproductive physiology facilitates great progress in aquaculture. Fish Physiol Biochem 28:493–497
Tanaka H, Nomura K, Yamamoto T, Oku H (2006) Development of artificial diets and rearing systems for eel larvae: the first success of production of glass eel in captivity. Bull Fish Res Agen Suppl 5:63–69 (in Japanese with English abstract)
Terahara T, Chow S, Kurogi H, Lee S-H, Tsukamoto K, Mochioka N, Tanaka H, Takeyama H (2011) Efficiency of peptide nucleic acid-directed PCR clamping and its application in the investigation of natural diets of the Japanese eel leptocephali. PLoS ONE 6:e25715
Tomoda T, Furuita H, Kamoshida M, Kurogi H, Shibuno T, Tanaka H, Tezuda N (2014) Nutritional enrichment trials of the minute rotifer Proales similis. J Fisheries Technol 6:179–184 (in Japanese with English abstract)
Tomoda T, Kurogi H, Okauchi M, Kamoshida M, Imaizumi H, Jinbo T, Nomura K, Furuita H, Tanaka H (2015) Hatchery-reared Japanese eel Anguilla japonica larvae ingest various organic matters formed part of marine snow. Nippon Suisan Gakkaishi 81:715–721 (in Japanese with English abstract)
Tomoda T, Chow S, Kurogi H, Okazaki M, Ambe D, Furuita H, Matsunari H, Nagai S, Yokouchi K, Nomura K, Tanaka H, Hasegawa D, Inaba N (2018) Observations of gut contents of anguilliform leptocephali collected in the western North Pacific. Nippon Suisan Gakkaishi 84:32–44 (in Japanese with English abstract)
Tsukamoto K, Yamada Y, Okamura A, Tanaka H, Miller MJ, Kaneko T, Horie N, Utoh T, Mikawa N, Tanaka S (2009) Positive buoyancy in eel leptocephali: an adaptation for life in the ocean surface layer. Mar Biol 156:835–846
Volkman JK, Tanoue E (2002) Chemical and biological studies of particulate organic matter in the ocean. J Oceanogr 58:265–279
Wakeham SG, Lee C (1989) Organic geochemistry of particulate matter in the ocean: the role of particles in oceanic sedimentary cycles. Organ Geochem 14:83–96
Westerberg H (1990) A proposal regarding the source of nutrition of leptocephalus larvae. Internat Revue ges Hydrobiol 75:863–864
Wilson SE, Steinberg DK, Buesseler KO (2008) Changes in fecal pellet characteristics with depth as indicators of zooplankton repackaging of particles in the mesopelagic zone of the subtropical and subarctic North Pacific Ocean. Deep-Sea Res II 55:1636–1647
Wullur S, Yoshimatsu T, Tanaka H, Ohtani M, Sakakura Y, Kim H-J, Hagiwara A (2013) Ingestion by Japanese eel Anguilla japonica larvae on various minute zooplanktons. Aquacult Sci 61:341–347
Yamada Y, Okamura A, Mikawa N, Utoh T, Horie N, Tanaka S, Miller MJ, Tsukamoto K (2009) Ontogenetic changes in phototaxis behavior during metamorphosis of artificially reared Anguilla japonica larvae. Mar Ecol Prog Ser 379:241–251
Yamada Y, Okamura A, Mikawa N, Horie N, Tsukamoto K (2019) A new liquid-type diet for leptocephali in mass production of artificial glass eels. Fish Sci 85:545–551
Acknowledgements
We would like to thank all the scientists who have studied the feeding ecology of leptocephali in the ocean and the laboratory whose research efforts are reviewed here. We especially appreciate the collaborations with our Japanese, French, and German colleagues for efforts to collect leptocephali and study various aspects of their feeding ecology, and we acknowledge the intensive research on artificially reared leptocephali conducted by scientists in Japan that are making the goal of producing glass eels from aquaculture a future possibility to help protect anguillid eel populations. This review paper and a companion review are designed in part to commemorate 20 years of research collaboration on eel larvae between the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published with support by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant no. JP19HP2002.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsukamoto, K., Miller, M.J. The mysterious feeding ecology of leptocephali: a unique strategy of consuming marine snow materials. Fish Sci 87, 11–29 (2021). https://doi.org/10.1007/s12562-020-01477-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-020-01477-3