Abstract
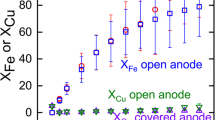
In the present study, copper (Cu) and iron boride (FexB) were produced for the first time through molten salt electrolysis using chalcopyrite (CuFeS2) in an oxide-based borax electrolyte. Molten salt electrolysis was carried out at 1073 K and a current density of 600 mA/cm2 for 3600 s under galvanostatic conditions. Cu and Fe/FexB were deposited on the graphite crucible surface used as the cathode. The particles obtained as a result of electrolysis were examined by X-ray diffraction (XRD) and determined to contain Cu and Fe/FexB. The location and ratio of Cu and FexB in the particles were investigated by using EDS mapping, energy-dispersive spectroscopy (EDS), and X-ray spectroscopy (XRD); Cu and Fe/FexB were found to be present throughout the particles at different ratios. Cu and Fe/FexB were successfully separated from each other by selective leaching of copper in a 1 M NH3–H2O solution. The time-dependent dissolution behavior of Cu was investigated at pH 8, 298 K, 600 rpm stirring rate for 900–5400 s, and it was observed that the dissolution rate increased over time and all the copper had completely dissolved after 5400 s. After leaching particles were examined by XRD and SEM, it was revealed that Fe/FexB particles did not contain any Cu.
Graphical Abstract










Similar content being viewed by others
References
Li Y, Kawashima N, Li J et al (2013) A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv Colloid Interface Sci 197–198:1–32. https://doi.org/10.1016/j.cis.2013.03.004
Baba AA, Ayinla KI, Adekola FA et al (2012) A review on novel techniques for chalcopyrite ore processing. Int J Min Eng Miner Process 1:1–16. https://doi.org/10.5923/j.mining.20120101.01
Schlesinger ME, King MJ, Sole KC, Davenport WG (2011) Direct-to-copper flash smelting. Extr Metall Copp. https://doi.org/10.1016/B978-0-08-096789-9.10010-1
Ruiz MC, Montes KS, Padilla R (2011) Chalcopyrite leaching in sulfate-chloride media at ambient pressure. Hydrometallurgy 109:37–42. https://doi.org/10.1016/j.hydromet.2011.05.007
Haver FP, Wong MM (1971) Recovery of copper, iron, and sulfur from chalcopyrite concentrate using a ferric chloride leach. J Met 23:25–29
Olvera OG, Rebolledo M, Asselin E (2016) Atmospheric ferric sulfate leaching of chalcopyrite: thermodynamics, kinetics and electrochemistry. Hydrometallurgy 165:148–158. https://doi.org/10.1016/j.hydromet.2015.09.017
Turan MD (2019) Optimization of selective copper extraction from chalcopyrite concentrate in presence of ammonium persulfate and ammonium hydroxide. Int J Miner Metall Mater 26:946–952. https://doi.org/10.1007/s12613-019-1804-y
Han B, Altansukh B, Haga K et al (2017) Leaching and kinetic study on pressure oxidation of chalcopyrite in H2SO4 solution and the effect of pyrite on chalcopyrite leaching. J Sustain Metall 3:528–542. https://doi.org/10.1007/s40831-017-0135-3
Biegler T, Constable DC (1977) Continuous electrolytic reduction of a chalcopyrite slurry. J Appl Electrochem 7:175–179. https://doi.org/10.1007/BF00611040
Habashi F (1997) Handbook of extractive metallurgy. Wiley-VCH, New York
Ge X, Wang X, Seetharaman S (2009) Copper extraction from copper ore by electro-reduction in molten CaCl2-NaCl. Electrochim Acta 54:4397–4402. https://doi.org/10.1016/j.electacta.2009.03.015
Wang D, Lu C, Zou X et al (2018) Electrolysis of converter matte in molten CaCl2-NaCl. J Mater Sci Chem Eng 06:1–11. https://doi.org/10.4236/msce.2018.62001
Xie H, Qu J, Ning Z et al (2018) Electrochemical co-desulfurization-deoxidation of low-grade nickel-copper matte in molten salts. J Electrochem Soc 165:E578–E583. https://doi.org/10.1149/2.1221811jes
Tan M, He R, Yuan Y et al (2016) Electrochemical sulfur removal from chalcopyrite in molten NaCl-KCl. Electrochim Acta 213:148–154. https://doi.org/10.1016/j.electacta.2016.07.088
Ge XL, Seetharaman S (2010) The salt extraction process—a novel route for metal extraction Part 2—Cu/Fe extraction from copper oxide and sulphides. Miner Process Extr Metall 119:93–100. https://doi.org/10.1179/174328510X498116
Free M, Moats M, Robinson T et al (2012) Electrometallurgy—now and in the future. Electrometallurgy 2012:1–27
Vignes A (2013a) Molten salt electrolysis operations. In: Vignes A (ed) Extractive metallurgy 3. Wiley, Berlin, pp 266–291
Chen GZ (2013) Forming metal powders by electrolysis. In: Isaac C (ed) Advances in powder metallurgy: properties, processing and applications. Woodhead Publishing Limited, Sawston, pp 19–41
Suzuki RO (2005) Calciothermic reduction of TiO2 and in situ electrolysis of CaO in the molten CaCl2. J Phys Chem Solids 66(2):461–465
Du C, Wang Z, Hou J et al (2014) Production of titanium powder by sodiothermic reduction in CaCl2 molten salts. Metall Mater Trans B 45:1750–1756. https://doi.org/10.1007/s11663-014-0083-2
Descallar-Arriesgado RF, Kobayashi N, Kikuchi T, Suzuki RO (2011) Calciothermic reduction of NiO by molten salt electrolysis of CaO in CaCl2melt. Electrochim Acta 56:8422–8429. https://doi.org/10.1016/j.electacta.2011.07.027
Xiao W, Wang D (2014) The electrochemical reduction processes of solid compounds in high temperature molten salts. Chem Soc Rev 43:3215–3228. https://doi.org/10.1039/c3cs60327j
Mohandas KS (2013) Direct electrochemical conversion of metal oxides to metal by molten salt electrolysis: a review. Miner Process Extr Metall 122:195–212. https://doi.org/10.1179/0371955313Z.00000000069
Abbasalizadeh A, Seetharaman S, Teng L et al (2013) Highlights of the salt extraction process. JOM. https://doi.org/10.1007/s11837-013-0752-7
Schulze R, Abbasalizadeh A, Bulach W et al (2018) An ex-ante LCA study of rare earth extraction from NdFeB magnet scrap using molten salt electrolysis. J Sustain Metall 4:493–505. https://doi.org/10.1007/s40831-018-0198-9
Abbasalizadeh A, Malfliet A, Seetharaman S et al (2017) Electrochemical extraction of rare earth metals in molten fluorides: conversion of rare earth oxides into rare earth fluorides using fluoride additives. J Sustain Metall 3:627–637. https://doi.org/10.1007/s40831-017-0120-x
Free M, Moats M, Houlachi G et al (2012) Electrometallurgy 2012. Wiley, Hoboken
Wang D, Gmitter AJ, Sadoway DR (2011) Production of oxygen gas and liquid metal by electrochemical decomposition of molten iron oxide. J Electrochem Soc 158:E51. https://doi.org/10.1149/1.3560477
Ozkalafat P, Kartal Sireli G, Timur S (2016) Electrodeposition of titanium diboride from oxide based melts. Surf Coatings Technol 308:128–135. https://doi.org/10.1016/j.surfcoat.2016.05.089
Vignes A (2013b) Molten salt electrolysis operations. In: Vignes A (ed) Extractive metallurgy 3. Wiley-ISTE, Hoboken, pp 265–291
Li G, Wang D, Jin X, Chen GZ (2007) Electrolysis of solid MoS2 in molten CaCl2 for Mo extraction without CO2 emission. Electrochem Commun 9:1951–1957. https://doi.org/10.1016/j.elecom.2007.05.007
Gao H, Tan M, Rong L et al (2014) Preparation of Mo nanopowders through electroreduction of solid MoS 2 in molten KCl–NaCl. Phys Chem Chem Phys 16:19514. https://doi.org/10.1039/C4CP01864H
Wang T, Gao H, Jin X et al (2011) Electrolysis of solid metal sulfide to metal and sulfur in molten NaCl-KCl. Electrochem Commun 13:1492–1495. https://doi.org/10.1016/j.elecom.2011.10.005
Sokhanvaran S, Lee S-K, Lambotte G, Allanore A (2016) Electrochemistry of molten sulfides: copper extraction from BaS-Cu2S. J Electrochem Soc 163:D115–D120. https://doi.org/10.1149/2.0821603jes
Sahu SK, Chmielowiec B, Allanore A (2017) Electrolytic extraction of copper, molybdenum and rhenium from molten sulfide electrolyte. Electrochim Acta 243:382–389. https://doi.org/10.1016/j.electacta.2017.04.071
Yin H, Chung B, Sadoway DR (2016) Electrolysis of a molten semiconductor. Nat Commun 7:1–5. https://doi.org/10.1038/ncomms12584
Sharma M, Ortlepp I, Bleck W (2019) Boron in heat-treatable steels: a review. Steel Res Int 90:1–28. https://doi.org/10.1002/srin.201900133
Gündüz İG, Ekerim A (2019) Production of ferroboron by using redesigned ESR process called electroslag melting for alloying by reductions (ESMAR). Mater Res Express 6:046508. https://doi.org/10.1088/2053-1591/aaf8aa
Mishra B, Olson DL (2005) Molten salt applications in materials processing. J Phys Chem Solids 66:396–401
Allanore A (2014) Features and challenges of molten oxide electrolytes for metal extraction. J Electrochem Soc. https://doi.org/10.1149/2.0451501jes
Kartal L, Daryal MB, Şireli GK, Timur S (2019) One-step electrochemical reduction of stibnite concentrate in molten borax. Int J Miner Metall Mater 26:1258–1265. https://doi.org/10.1007/s12613-019-1867-9
Kartal L, Timur S (2019) Direct electrochemical reduction of copper sulfide in molten borax. Int J Miner Metall Mater 26:992–998. https://doi.org/10.1007/s12613-019-1821-x
Kartal G, Timur S (2013) Growth kinetics of titanium borides produced by CRTD-Bor method. Surf Coatings Technol 215:440–446. https://doi.org/10.1016/j.surfcoat.2012.08.076
Radmehr V, Koleini SMJ, Khalesi MR, Tavakoli Mohammadi MR (2013) Ammonia leaching: a new approach of copper industry in hydrometallurgical processes. J Inst Eng Ser D 94:95–104. https://doi.org/10.1007/s40033-013-0029-x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was U. Pal.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kartal, L., Daryal, M.B. & Timur, S. A New Approach for Cu and Fe/FexB Production from Chalcopyrite by Molten Salt Electrolysis. J. Sustain. Metall. 6, 751–760 (2020). https://doi.org/10.1007/s40831-020-00312-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-020-00312-4