Abstract
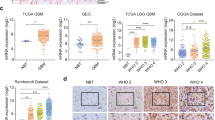
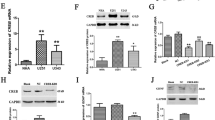
Abnormally high expression of glial cell line-derived neurotrophic factor (GDNF) derived from glioma cells has essential impacts on gliomagenesis and development, but the molecular basis underlying increased GDNF expression in glioma cells remain unclear. This work aimed to study the molecular mechanisms that may explain the accumulation of GDNF in glioma. Firstly, we observed that cAMP response element-binding protein (CREB), known as an important transcription factor for binding of GDNF promoter region, was highly expressed with an apparent accumulation into the nucleus of glioma cells, which may contribute to the transcription of GDNF. Secondly, CUE domain-containing protein 2 (CUEDC2), a ubiquitin-regulated protein, could increase the amount of binding between the E3 ligase tripartite motif-containing 21 (TRIM21) and CREB and affect the CREB level. Like our previous study, it showed that there was a significantly down-regulation of CUEDC2 in glioma. Finally, our data suggest that GDNF expression is indirectly regulated by transcription factor ubiquitination. Indeed, down-regulation of CUEDC2, decreased the ubiquitination and degradation of CREB, which was associated to high levels of GDNF. Furthermore, abundant CREB involved in the binding to the GDNF promoter region contributes to GDNF high expression in glioma cells. Collectively, it was verified the GDNF expression was affected by CREB ubiquitination regulated by CUEDC2 level.





Similar content being viewed by others
Abbreviations
- GDNF:
-
Glial cell line-derived neurotrophic factor
- CUEDC2:
-
CUE domain-containing protein 2
- TRIM21:
-
Tripartite motif-containing 21
- CREB:
-
CAMP response element-binding protein
- ChIP-PCR:
-
Chromatin immunoprecipitation-polymerase chain reaction
- HA:
-
Human astrocytes
References
Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260(5111):1130–1132
Ku MC, Wolf SA, Respondek D, Matyash V, Pohlmann A, Waiczies S, Waiczies H, Niendorf T, Synowitz M, Glass R, Kettenmann H (2013) GDNF mediates glioblastoma-induced microglia attraction but not astrogliosis. Acta Neuropathol 125(4):609–620. https://doi.org/10.1007/s00401-013-1079-8
Lu DY, Leung YM, Cheung CW, Chen YR, Wong KL (2010) Glial cell line-derived neurotrophic factor induces cell migration and matrix metalloproteinase-13 expression in glioma cells. Biochem Pharmacol 80(8):1201–1209. https://doi.org/10.1016/j.bcp.2010.06.046
Ng WH, Wan GQ, Peng ZN, Too HP (2009) Glial cell-line derived neurotrophic factor (GDNF) family of ligands confer chemoresistance in a ligand-specific fashion in malignant gliomas. J Clin Neurosci 16(3):427–436. https://doi.org/10.1016/j.jocn.2008.06.002
Wiesenhofer B, Stockhammer G, Kostron H, Maier H, Hinterhuber H, Humpel C (2000) Glial cell line-derived neurotrophic factor (GDNF) and its receptor (GFR-alpha 1) are strongly expressed in human gliomas. Acta Neuropathol 99(2):131–137
Yu ZQ, Zhang BL, Ren QX, Wang JC, Yu RT, Qu DW, Liu ZH, Xiong Y, Gao DS (2013) Changes in transcriptional factor binding capacity resulting from promoter region methylation induce aberrantly high GDNF expression in human glioma. Mol Neurobiol 48(3):571–580. https://doi.org/10.1007/s12035-013-8443-5
Zhang L, Wang D, Han X, Tang F, Gao D (2019) Mechanism of methylation and acetylation of high GDNF transcription in glioma cells: a review. Heliyon 5(6):e01951
Zhang B, Gu X, Han X, Gao Q, Liu J, Guo T, Gao D (2020) Crosstalk between DNA methylation and histone acetylation triggers GDNF high transcription in glioblastoma cells. Clin Epigenetics 12:47. https://doi.org/10.1186/s13148-020-00835-3
Firestein R, Feuerstein N (1998) Association of activating transcription factor 2 (ATF2) with the ubiquitin-conjugating enzyme hUBC9. Implication of the ubiquitin/proteasome pathway in regulation of ATF2 in T cells. J Biol Chem 273(10):5892–5902
Fuchs SY, Ronai Z (1999) Ubiquitination and degradation of ATF2 are dimerization dependent. Mol Cell Biol 19(5):3289–3298
Sutter CH, Laughner E, Semenza GL (2000) Hypoxia-inducible factor 1alpha protein expression is controlled by oxygen-regulated ubiquitination that is disrupted by deletions and missense mutations. Proc Natl Acad Sci USA 97(9):4748–4753. https://doi.org/10.1073/pnas.080072497
Yaron A, Hatzubai A, Davis M, Lavon I, Amit S, Manning AM, Andersen JS, Mann M, Mercurio F, Ben-Neriah Y (1998) Identification of the receptor component of the IkappaBalpha-ubiquitin ligase. Nature 396(6711):590–594. https://doi.org/10.1038/25159
Taylor CT, Furuta GT, Synnestvedt K, Colgan SP (2000) Phosphorylation-dependent targeting of cAMP response element binding protein to the ubiquitin/proteasome pathway in hypoxia. Proc Natl Acad Sci USA 97(22):12091–12096. https://doi.org/10.1073/pnas.220211797
Zhang PJ, Zhao J, Li HY, Man JH, He K, Zhou T, Pan X, Li AL, Gong WL, Jin BF, Xia Q, Yu M, Shen BF, Zhang XM (2007) CUE domain containing 2 regulates degradation of progesterone receptor by ubiquitin-proteasome. EMBO J 26(7):1831–1842. https://doi.org/10.1038/sj.emboj.7601602
Donaldson KM, Yin H, Gekakis N, Supek F, Joazeiro CA (2003) Ubiquitin signals protein trafficking via interaction with a novel ubiquitin binding domain in the membrane fusion regulator, Vps9p. Curr Biol CB 13(3):258–262
Man J, Zhang X (2011) CUEDC2: an emerging key player in inflammation and tumorigenesis. Protein Cell 2(9):699–703. https://doi.org/10.1007/s13238-011-1089-z
Jian Z, Liang B, Pan X, Xu G, Guo SS, Li T, Zhou T, Xiao YB (2016) CUEDC2 modulates cardiomyocyte oxidative capacity by regulating GPX1 stability. EMBO Mol Med 8(7):813–829. https://doi.org/10.15252/emmm.201506010
Li F, Tang C, Jin D, Guan L, Wu Y, Liu X, Wu X, Wu QY, Gao D (2017) CUEDC2 suppresses glioma tumorigenicity by inhibiting the activation of STAT3 and NF-kappaB signaling pathway. Int J Oncol 51(1):115–127. https://doi.org/10.3892/ijo.2017.4009
Woodbury D, Schaar DG, Ramakrishnan L, Black IBJBR (1998) Novel structure of the human GDNF gene. Brain Res 803(1–2):95–104
Wan G, Too HP (2010) A specific isoform of glial cell line-derived neurotrophic factor family receptor alpha 1 regulates RhoA expression and glioma cell migration. J Neurochem 115(3):759–770. https://doi.org/10.1111/j.1471-4159.2010.06975.x
Xiong Y, Liu L, Zhu S, Zhang B, Qin Y, Yao R, Zhou H, Gao DS (2017) Precursor N-cadherin mediates glial cell line-derived neurotrophic factor-promoted human malignant glioma. Oncotarget 8(15):24902–24914. https://doi.org/10.18632/oncotarget.15302
Montminy MR, Bilezikjian LM (1987) Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature 328(6126):175–178. https://doi.org/10.1038/328175a0
Yamamoto KK, Gonzalez GA, Biggs WH 3rd, Montminy MR (1988) Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature 334(6182):494–498. https://doi.org/10.1038/334494a0
Mayr B, Montminy M (2001) Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2(8):599–609. https://doi.org/10.1038/35085068
Shaywitz AJ, Greenberg ME (1999) CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 68:821–861. https://doi.org/10.1146/annurev.biochem.68.1.821
Shukla A, Bosenberg MW, MacPherson MB, Butnor KJ, Heintz NH, Pass HI, Carbone M, Testa JR, Mossman BT (2009) Activated cAMP response element binding protein is overexpressed in human mesotheliomas and inhibits apoptosis. Am J Pathol 175(5):2197–2206. https://doi.org/10.2353/ajpath.2009.090400
Golan M, Schreiber G, Avissar S (2011) Antidepressants elevate GDNF expression and release from C(6) glioma cells in a beta-arrestin1-dependent, CREB interactive pathway. Int J Neuropsychopharmacol 14(10):1289–1300. https://doi.org/10.1017/s1461145710001550
Hisaoka K, Maeda N, Tsuchioka M, Takebayashi M (2008) Antidepressants induce acute CREB phosphorylation and CRE-mediated gene expression in glial cells: a possible contribution to GDNF production. Brain Res 1196:53–58. https://doi.org/10.1016/j.brainres.2007.12.019
Daniel P, Filiz G, Brown DV, Hollande F, Gonzales M, D'Abaco G, Papalexis N, Phillips WA, Malaterre J, Ramsay RG, Mantamadiotis T (2014) Selective CREB-dependent cyclin expression mediated by the PI3K and MAPK pathways supports glioma cell proliferation. Oncogenesis 3:e108. https://doi.org/10.1038/oncsis.2014.21
Perry C, Sklan EH, Soreq H (2004) CREB regulates AChE-R-induced proliferation of human glioblastoma cells. Neoplasia 6(3):279–286. https://doi.org/10.1593/neo.3424
Tan X, Wang S, Yang B, Zhu L, Yin B, Chao T, Zhao J, Yuan J, Qiang B, Peng X (2012) The CREB-miR-9 negative feedback minicircuitry coordinates the migration and proliferation of glioma cells. PLoS ONE 7(11):e49570. https://doi.org/10.1371/journal.pone.0049570
Tan X, Wang S, Zhu L, Wu C, Yin B, Zhao J, Yuan J, Qiang B, Peng X (2012) cAMP response element-binding protein promotes gliomagenesis by modulating the expression of oncogenic microRNA-23a. Proc Natl Acad Sci USA 109(39):15805–15810. https://doi.org/10.1073/pnas.1207787109
Lee JH, Liu R, Li J, Zhang C, Wang Y, Cai Q, Qian X, Xia Y, Zheng Y, Piao Y, Chen Q, de Groot JF, Jiang T, Lu Z (2017) Stabilization of phosphofructokinase 1 platelet isoform by AKT promotes tumorigenesis. Nat Commun 8(1):949. https://doi.org/10.1038/s41467-017-00906-9
Foltz C, Napolitano A, Khan R, Clough B, Hirst EM, Frickel EM (2017) TRIM21 is critical for survival of Toxoplasma gondii infection and localises to GBP-positive parasite vacuoles. Sci Rep 7(1):5209. https://doi.org/10.1038/s41598-017-05487-7
Zhao Z, Wang Y, Yun D, Huang Q, Meng D, Li Q, Zhang P, Wang C, Chen H, Lu D (2020) TRIM21 overexpression promotes tumor progression by regulating cell proliferation, cell migration and cell senescence in human glioma. Am J Cancer Res 10(1):114–130
Müller J, Maurer V, Reimers K, Vogt P, Bucan V (2015) TRIM21, a negative modulator of LFG in breast carcinoma MDA-MB-231 cells in vitro. Int J Oncol 47(5):1634–1646
Si W, Zhou J, Zhao Y, Zheng J, Cui L (2020) SET7/9 promotes multiple malignant processes in breast cancer development via RUNX2 activation and is negatively regulated by TRIM21. Cell Death Dis 11(2):151. https://doi.org/10.1038/s41419-020-2350-2
Zhou W, Zhang Y, Zhong C, Hu J, Hu H, Zhou D, Cao M (2018) Decreased expression of TRIM21 indicates unfavorable outcome and promotes cell growth in breast cancer. Cancer Manag Res 10:3687–3696. https://doi.org/10.2147/CMAR.S175470
Acknowledgements
We are grateful for glioma samples provided by the Affiliated Hospital of Xuzhou Medical University. We would like to acknowledge the Department of Anatomy and Neurobiology of Xuzhou Medical University.
Funding
This study was funded by the National Natural Science Research Foundation of China (nos. 81602464, 81772688), Six Talent Peaks in Jiangsu Province Jiangsu (SWYY-088), Qing Lan Project in Jiangsu Province (2017 to B.L. Zhang), and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
Z-BL and D-SG made substantial contributions to conception and design, funding, and project supervision. X-FL and C-XT conceived the study, performed most experiments, analyzed data, and wrote the manuscript. LZ and S-YT performed and checked the experiments, integrated data. AA Abdulrahman embellished the paper. YW, G-QJ and YG were responsible for statistical analysis and organizing pictures and tables. All authors read approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared no potential conflict of interest.
Ethical Approval
Glioma tissues were obtained from the Affiliated Hospital of Xuzhou Medical University. All experimental protocols were approved by the Clinical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University. All participates received and signed informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11064_2020_3140_MOESM1_ESM.tif
Supplementary Fig. 1. Prediction of Lysine ubiquitylation for CREB by UbPred using the CREB amino acid sequence.Supplementary file1 (TIF 7705 kb)
11064_2020_3140_MOESM2_ESM.tif
Supplementary Fig. 2. The original gel of complexes with the CUEDC2 antibody used for mass spectrometry.Supplementary file2 (TIF 4322 kb)
11064_2020_3140_MOESM3_ESM.tif
Supplementary Fig. 3. Transfected U251 cells were observed under the fluorescence microscope. The upper picture showed that transfected U251 cells were observed under a light microscope. The middle picture showed that transfected U251 cells were observed under the green fluorescence. Merge picture showed at the lowest picture. Supplementary file3 (TIF 14207 kb)
Rights and permissions
About this article
Cite this article
Liu, XF., Tang, CX., Zhang, L. et al. Down-Regulated CUEDC2 Increases GDNF Expression by Stabilizing CREB Through Reducing Its Ubiquitination in Glioma. Neurochem Res 45, 2915–2925 (2020). https://doi.org/10.1007/s11064-020-03140-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03140-w