Abstract
A novel microwave-assisted sol–gel-hydrothermal method was employed to rapidly synthesize Ba0.85Ca0.15Zr0.1Ti0.9O3 (BCZT) powders. The effects of reaction time on the structure, crystallinity, purity and morphology of the products were investigated. The results of XRD, FTIR, SEM and TEM indicated that BCZT powders could be obtained in even 60 min at a low synthesis temperature of 180 °C, which were well-crystallized with stoichiometric composition and uniform grain size (~ 85 nm). BCZT ceramic derived from the rapidly-synthesized powders had a dense microstructure and good electrical properties (εm = 9579, d33 = 496 pC/N, 2Pr = 25.22 µC/cm2, 2Ec = 7.52 kV/cm). The significant electrical properties were closely related to the high activity of the BCZT powders, resulting from the rapid microwave-assisted sol–gel-hydrothermal process.
Similar content being viewed by others
Introduction
In the past decades, lead-based materials have been widely used in piezoelectric or ferroelectric fields due to the significant electrical performance. Unfortunately, their applications are being limited worldwide because of the toxicity of lead as well as the related health and environmental issues1. As a result, a series of lead-free piezoelectric materials have been developed and paid more and more attention. Among them, Ba0.85Ca0.15Zr0.1Ti0.9O3 (hereinafter referred to as BCZT) is found to be a lead-free piezoelectric material with excellent electrical properties2, which is even comparable to the lead-based materials including the most popular PZT3.
As a complex system, the performance of BCZT is dependent largely on the purity, stoichiometry and grain size of the BCZT powders synthesized by different methods. Also, for the preparation of BCZT ceramics, calcination and sintering both require high temperatures. Although most literature reports the preparation of BCZT powder the by solid-state reaction method, relatively higher calcination temperature (> 1300 °C) is necessary4,5. Thus, liquid-phase powder precursor synthesis methods deserve study in order to decrease calcination temperature. Even though several liquid-phase synthesis techniques such as sol–gel process, oxalate precursor route and citrate method have been tried to solve the above problems, the issues like high calcination temperature (> 800 °C) still exist6,7,8,9. Besides, hydrothermal reaction is used to prepare BCZT powders, and the synthesis temperature can be decreased to below 200 °C. Nevertheless, purity issues still limit the popularization of the method, for impurities induced from the complex hydrothermal reaction10,11.
Recently, a sol–gel-hydrothermal technique has been reported for powder preparation12,13,14, which combines both the advantages of sol–gel and hydrothermal methods so that the powders with high purity and homogeneity could be obtained at a rather low temperature (< 200 °C). Nevertheless, long reaction time (> 12 h) in the sol–gel-hydrothermal process is still needed13.
Microwave-assisted sol–gel-hydrothermal method (hereinafter referred to as MSGH) is a novel technique that can synthesize powders rapidly at low temperature. Based on the sol–gel-hydrothermal process which has been used to synthesis homogeneous and highly pure nano-powders at low temperature15, the introduction of microwave may enhance the sol–gel-hydrothermal reaction efficiency and shorten the synthesis time greatly, so that BCZT powders with high purity, uniform grain size and high activity could be well produced16. Unfortunately, at the best of our knowledge, rapid synthesizing BCZT powders at low temperatures by MSGH was never reported.
In the present study, a novel technique, MSGH, was employed to rapidly synthesize crystalline BCZT powders with high purity and activity rapidly (60 min) at low temperature (180 °C) for the first time. The effects of reaction time on the structure, crystallinity, purity and morphology of BCZT powders were studied. Furthermore, followed by traditional sintering method, electrical properties of MSGH derived BCZT ceramics were measured, so as to verify the high activity of the powders.
Experimental
Ba0.85Ca0.15Zr0.1Ti0.9O3 (BCZT) powders were synthesized by the microwave-assisted sol–gel-hydrothermal method. Firstly, BCZT gel was formed through sol–gel process by using barium acetate (BaC4H6O4), calcium acetate (CaC4H6O4), titanium butoxide (TiC16H36O4) and zirconium butoxide solution (ZrC16H36O4) as the raw materials. The acetic acid solution was mixed with the raw materials and kept stirring at 60 °C for 30 min. The solution turned into sol and then transformed to gel. After dried overnight and grounded, the formed gel was introduced to a NaOH aqueous solution with a concentration of 4 M. The precursor solutions were then sealed and placed in the microwave hydrothermal equipment (JUPITER BF, SINEO, China). The reaction was carried out under 300 W microwave and a synthesis temperature of 180 °C. The precipitate was centrifuged and washed with distilled water and absolute ethanol for several times to remove the soluble impurities. After drying, the BCZT powders were obtained. Finally, the MSGH-derived BCZT powders were pressed into disks, and further sintered at 1400 °C for 2 h to produce BCZT ceramics by traditional sintering method.
The phase structure of the samples was identified by XRD (Rigaku, Ultima III) with Cu-Kα radiation, FTIR (Thermo, Nicolet-6700) with the KBr in the range from 400 to 4000 cm−1 and Raman spectra (Renishaw, INVIA), respectively. The microstructure was characterized by SEM (Quanta, FEG250) and TEM (JEOL, JEM-2100F). The piezoelectric and dielectric properties were measured by quasi-static d33 meter (Institute of Acoustics, ZJ-3AN) and precision LCR meter (Agilent, E4980A). The ferroelectric property was obtained by a ferroelectric test system using a precision LC unit (Radiant, Premier II) at room temperature with a frequency of 10 Hz.
Results and discussion
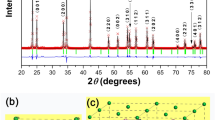
Figure 1 shows the XRD patterns of BCZT powders synthesized at 180 °C for various reaction time of 15 min, 30 min, 45 min and 60 min. It can be seen that all the powders exhibit perovskite structure with no impurities, suggesting a solid solution formed by doping Ca and Zr into BaTiO3 lattice17. The inset of Fig. 1 shows the FWHM and powder size of the BCZT powders as a function of reaction time, calculated from the XRD data. Clearly, a better crystallinity could be observed with increasing the reaction time, since the value of FWMH decreases gradually. Also, the powder size calculated by Scherrer formula is estimated to be 29 nm, 46 nm, 59 nm and 78 nm, respectively.
Figure 2 shows the FTIR spectra of the BCZT powders synthesized at 180 °C for various reaction time of 15 min, 30 min, 45 min and 60 min. No obvious impurity peaks corresponding to CO32− (around 690, 860 and 1750 cm−1) or X–O–C (X = Ti, Zr) groups (around 1120 cm−1) can be noted with the extending reaction time18,19,20. Meanwhile, for all the samples, the absorption peaks corresponding to O–H (around 1640 cm−1 and 3000–3600 cm−1) as well as –COOH (around 1420 cm−1) disappear nearly and the peaks corresponding to Z–O (Z = Ba, Ca, Ti, Zr) were quite significant13, implying the high purity and crystallinity of MSGH derived BCZT powders12.
Figure 3 shows the SEM images of BCZT powders synthesized at 180 °C for various reaction time of 15 min, 30 min, 45 min and 60 min. For 15 min and 30 min samples, blurred boundaries can be observed with the powder sizes of about 20 nm and 50 nm respectively. With the extending reaction time to 45 min, the powder size increases to about 65 nm. When the reaction time extends to 60 min, the agglomeration is improved and the powder size increases to about 85 nm. The powder size of SEM is close to the calculated value from XRD.
Figure 4 shows the TEM images of BCZT powders obtained by MSGH at 180 °C for 60 min. It is clear from Fig. 4a that the boundaries of the particulates are clear and the powder size from TEM image is around 90 nm, which is basically consistent with SEM. Moreover, the powder size obtained here is slightly smaller compared with our previous work (around 105 nm by sol–gel method and 93 nm by sol–gel-hydrothermal method15,21) and other literature (around 100 nm by hydrothermal method22). Furthermore, from Fig. 4b, fringe features of crystal lattice with a fringe spacing of 0.287 nm corresponding to (110) planes can be observed clearly, proving the good crystallinity of the powders synthesized at 180 °C for 60 min7.
Compared with our previous work about low temperature synthetizing BCZT powders by sol–gel-hydrothermal method, MSGH can shorten the reaction time greatly (about 11 h shorter)15, which can be attributed to the introduction of microwave in enhancing the reaction efficiency16. In addition, compared with other conventional methods, MSGH has a shorter reaction time than solid-state reaction (> 2 h), sol–gel method (> 2 h) and hydrothermal method (> 10 h)2,7,8, indicating the advantages of MSGH in rapid synthesis of powders.
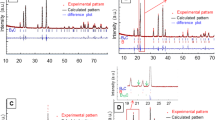
BCZT powders synthesized at 180 °C for 60 min were further sintered at 1400 °C to prepare BCZT ceramics. Figure 5 shows the XRD patterns of BCZT ceramic. All the diffraction peaks show a typical perovskite structure with no impurities, suggesting the high purity of the BCZT ceramic23,24. Besides, the sharp diffraction peaks of the XRD pattern indicate great crystallinity of BCZT ceramic sintered at 1400 °C.
In order to confirm the phase structures of the obtained BCZT ceramics, Raman spectra are measured ranging from 125 cm−1 to 775 cm−1, which is shown in Fig. 6. Modes at 149, 197, 292, 525, 730 cm−1 can be clearly observed respectively. Modes at about 149 and 197 cm−1 reflect the existence of the rhombohedral phase25, while modes at about 292, 525 and 730 cm−1 prove the existence of the tetragonal phase26, which agrees well with previous reports27,28. Thus, the result of Raman spectra indicates he coexistence of two phases in MPB structure of BCZT ceramics.
Figure 7 shows the SEM image and EDS spectra of BCZT ceramics. The SEM image shows a homogenous distribution of grains with a dense microstructure and the grain size is measured to be 20–30 μm. The density of the obtained BCZT ceramics is 5.57 g/cm3, which is better than other related reports29,30. As shown in the EDS spectrums, all elements belonging to BCZT ceramics are uniformly distributed throughout the observed area, without any significant element enrichment areas. Compared with solid-state reaction and sol-gel derived BCZT ceramics, MSGH derived BCZT ceramics have a lower sintering temperature (1400 °C) than those in the related reports (1450–1600 °C)7,31,32,33, which may be attributed to the high activity of BCZT powders prepared by MSGH34,35.
Figure 8a shows the temperature vs. dielectric constant (εr) for BCZT ceramics measured at 1 kHz, 10 kHz, 100 kHz and 1000 kHz, respectively. The TC and εm is measured to be 83.61 °C and 9579 respectively under 1 kHz frequency, and the εm obtained here is slightly higher than that in other related reports8,36. However, an interesting phenomenon can be observed that TC measured in this work is lower than that in other literature3,7, which may be attributed to the larger grain size 37. Duo to the ferroelectric transition of BCZT ceramics, two dielectric anomalies can be observed, which is related to the phase transition of rhombohedral-tetragonal and tetragonal-cubic2. Moreover, a relaxor ferroelectrics phenomenon of strong frequency dispersion and diffuse phase transition could be observed clearly, which is manifested as that not only εr decreases but also TC moves to higher temperatures area with the increasing frequency31. Furthermore, the temperature and frequency—dependence of dielectric loss (tanδ) of the BCZT ceramic is also shown in the Fig. 8a. The relatively low tanδ observed in this work may be ascribed to the less cavities in the dense BCZT ceramic and the lower electron diffusion in the grain boundaries38,39.
It is known that the dielectric constant of a normal ferroelectric above the Curie temperature follows the Curie–Weiss law which can be described by:
where T0 is the Curie–Weiss temperature and C is the Curie–Weiss constant.
Figure 8b shows the plots of temperature vs. dielectric constant (104/εr at 100 kHz) fitted to the Curie–Weiss law. For BCZT ceramics obtained through MSGH, T0 = 83.45 °C and C = 1.33 × 105 °C were obtained. The C value obtained here is close to the existing literature on BaTiO3 (approximately 105 °C), indicates a displacive type phase transition in BCZT ceramics32. Moreover, a deviation of dielectric constant from the Curie–Weiss law starting at TC can be seen. The parameter ΔTm which is often used to characterized the degree of the deviation from the Curie–Weiss law and a relaxor-like behavior can be defined as32,40:
where TCW denotes the temperature at which the dielectric constant starts to deviate from the Curie–Weiss law and Tm represents the temperature at which dielectric constant reaches its maximum. A narrower dielectric peak of BCZT ceramic which indicates a weaker diffuse phase transition behavior can be clearly observed as the value of ΔTm (29.54 °C) is lower than that in other literature41.
To further describe dielectric behavior of BCZT ceramic, a modified empirical expression proposed by Uchino and Nomura can be given as42:
where C is the Curie–Weiss constant, and γ is a constant implying the degree of diffuse phase transition (1 < γ < 2). A normal Curie–Weiss ferroelectrics can be observed at γ = 1 and an ideal relaxor ferroelectric can be observed at γ = 2. The inset in Fig. 8b shows the plot of ln (T − Tm) vs. ln (1/εr—1/εm). Depending on the slope of the fitting curve, the value of γ is fitted to be 1.524, reflecting a characteristic of relaxor ferroelectric to some degree.
The P-E hysteresis loops of the BCZT ceramics measured under different electric fields are shown in Fig. 9. The inset shows electric fields vs. remnant polarization (2Pr) and coercive field (2Ec). With the increasing electrical fields, 2Pr as well as 2Ec increases gradually and the hysteresis loops tend to be saturated. Well saturated loops with large 2Pr (25.22 µC/cm2) and moderate 2Ec (7.52 kV/cm) were obtained under 30 kV/cm electric field.
Immersed in a silicone oil bath, the BCZT samples coated with silver electrodes were subjected to electrical poling under a certain poling condition (poling field = 35 kV/cm, poling temperature = 300 K, poling time = 30 min). After poling process, the piezoelectric coefficient (d33) of the BCZT ceramics are measured, giving the value of 496 pC/N. The large piezoelectric property may be related to the MPB structure in BCZT ceramic and the release of the stress in the domains wall. This may promote the lateral movement of domain walls, even the reorientation and the growth of domains during poling process43.
Compare with other reports, MSGH derived BCZT ceramics with better electrical properties (4.66 ~ 23.38 µC/cm27,44, 164 ~ 424 pC/N5,45,46) can be obtained at a relatively lower sintering temperature (above 1450 °C5,7,47), which may attribute to the high activity of BCZT powders prepared by MSGH in this work22.
Conclusion
In summary, Ba0.85Ca0.15Zr0.1Ti0.9O3 (BCZT) powders were synthesized rapidly by a novel microwave-assisted sol–gel-hydrothermal method (MSGH). The reaction time was shortened to 60 min even at a lower synthesis temperature of 180 °C, as compared with the sol–gel process or sol–gel-hydrothermal methods. BCZT powders were well-crystallized and compositional uniform with fine grains (~ 85 nm). The BCZT ceramic derived from the MSGH-synthesized powders had a dense structure (density 5.57 g/cm3) as well as excellent electrical properties (εm = 9579, d33 = 496 pC/N, 2Pr = 25.22 µC/cm2, Ec = 7.52 kV/cm), which was attributed to the high activity of the powders rapidly synthesized by MSGH.
References
Liu, X. et al. Enhancing piezoelectric properties of BCZT ceramics by Sr and Sn co-doping. J. Alloys Compd. 640(15), 128–133 (2015).
Liu, W. & Ren, X. Large piezoelectric effect in Pb-free ceramics. Phys. Rev. Lett. 103(25), 257602 (2009).
Hao, J., Bai, W., Li, W. & Zhai, J. Correlation between the microstructure and electrical properties in high-performance (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 lead-free piezoelectric ceramics. J. Am. Ceram. Soc. 95(6), 1998–2006 (2012).
Wu, J. et al. Role of room-temperature phase transition in the electrical properties of (Ba, Ca)(Ti, Zr)O3 ceramics. Scripta Mater. 65(9), 771–774 (2011).
Wu, J. et al. Composition and poling condition-induced electrical behavior of (Ba0.85Ca0.15)(Ti1-xZrx)O3 lead-free piezoelectric ceramics. J. Eur. Ceram. Soc. 32(4), 891–898 (2012).
Liu, X. et al. Enhancing pyroelectric properties of Li-doped (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 lead-free ceramics by optimizing calcination temperature. Jpn. J. Appl. Phys. 54(7), 071501 (2015).
Praveen, J. P. et al. Large piezoelectric strain observed in sol-gel derived BZT-BCT ceramics. Curr. Appl. Phys. 14(3), 396–402 (2014).
Puli, V. S. et al. Barium zirconate-titanate/barium calcium-titanate ceramics via sol-gel process: novel high-energy-density capacitors. J. Phys. D Appl. Phys. 44(39), 395403 (2011).
Bharathi, P. & Varma, K. B. R. Grain and the concomitant ferroelectric domain size dependent physical properties of Ba0.85Ca0.15Zr0.1Ti0.9O3 ceramics fabricated using powders derived from oxalate precursor route. J. Appl. Phys. 116(16), 164107 (2014).
Liu, Y., Pu, Y. & Sun, Z. Enhanced relaxor ferroelectric behavior of BCZT lead-free ceramics prepared by hydrothermal method. Mater. Lett. 137(15), 128–131 (2014).
Ji, W. et al. Tailoring structure and performance of BCZT ceramics prepared via hydrothermal method. Phys. B 567(3), 65–78 (2019).
Wang, W., Cao, L., Liu, W., Su, G. & Zhang, W. Low-temperature synthesis of BaTiO3 powders by the sol-gel-hydrothermal method. Ceram. Int. 39(6), 7127–7134 (2013).
Hanani, Z., Ablouh, E. H., Mezzane, D., Fourcade, S. & Goune, M. Very-low temperature synthesis of pure and crystalline lead-free Ba0.85Ca0.15Zr0.1Ti0.9O3 ceramic. Ceram. Int. 44(9), 10997–11000 (2018).
Song, Z. Q. et al. Synthesis of manganese titanate MnTiO3 powders by a sol–gel-hydrothermal method. Mater. Sci. Eng. B-Adv. 113(2), 121–124 (2004).
Ji, X. et al. Structural and electrical properties of BCZT ceramics synthesized by sol–gel-hydrothermal process at low temperature. J. Mater. Sci. Mater. Electron. 30(13), 12197–12203 (2019).
Yu, H., Zhu, Y. J. & Lu, B. Q. Highly efficient and environmentally friendly microwave-assisted hydrothermal rapid synthesis of ultralong hydroxyapatite nanowires. Ceram. Int. 44(11), 12352–12356 (2018).
Kaddoussi, H. et al. Sequence of structural transitions and electrocaloric properties in (Ba1-xCax)(Zr0.1Ti0.9)O3 ceramics. J. Alloys Compd. 713(5), 164–179 (2017).
Thongtem, T., Tipcompor, N., Phuruangrat, A. & Thongtem, S. Characterization of SrCO3 and BaCO3 nanoparticles synthesized by sonochemical method. Mater. Lett. 64(4), 510–512 (2010).
Darwish, A., Badr, Y., El Shaarawy, M., Shash, N. & Battisha, I. Influence of the Nd3+ ions content on the FTIR and the visible up-conversion luminescence properties of nano-structure BaTiO3, prepared by sol-gel technique. J. Alloys Compd. 489(2), 451–455 (2010).
Harizanov, O., Harizanova, A. & Ivanova, T. Formation and characterization of sol–gel barium titanate. Mater. Sci. Eng. B Adv. 106(2), 191–195 (2004).
Ji, X. et al. Structural and electrical properties of BCZT ceramics synthesized by sol-gel process. J. Mater. Sci. Mater. Electron. 29(9), 7592–7599 (2018).
Fang, B. et al. Decreasing sintering temperature for BCZT lead-free ceramics prepared via hydrothermal route. Funct. Mater. Lett. 10(4), 1750046 (2017).
Li, S., Wang, C., Ji, X., Shen, Q. & Zhang, L. Effect of composition fluctuation on structural and electrical properties of BZT-xBCT ceramics prepared by plasma activated sintering. J. Eur. Ceram. Soc. 37(5), 2067–2072 (2017).
Xue, D. et al. Elastic, piezoelectric, and dielectric properties of Ba(Zr0.2Ti0.8)O3-50(Ba0.7Ca0.3)TiO3 Pb-free ceramic at the morphotropic phase boundary. J. Appl. Phys. 109(5), 054110 (2011).
Bijalwan, V. et al. The effect of sintering temperature on the microstructure and functional properties of BCZT-xCeO2 lead free ceramics. Mater. Res. Bull. 141, 121–129 (2019).
Li, S., Wang, C., Li, L., Shen, Q. & Zhang, L. Effect of annealing temperature on structural and electrical properties of BCZT ceramics prepared by plasma activated sintering. J. Alloys Compd. 730(5), 182–190 (2018).
Zhao, L., Zhang, B. P., Zhou, P. F., Zhu, L. F. & Li, J. F. Effect of Li2O addition on sintering and piezoelectric properties of (Ba, Ca)(Ti, Sn)O3 lead-free piezoceramics. J. Eur. Ceram. Soc. 35(2), 533–540 (2015).
Miao, S. et al. Polar order and diffuse scatter in Ba(Ti1-xZrx)O3 ceramics. J. Appl. Phys. 106(11), 114111 (2009).
Li, W., Xu, Z., Chu, R., Fu, P. & Zang, G. High piezoelectric d33 coefficient of lead-free (Ba0.93Ca0.07)(Ti0.95Zr0.05)O3 ceramics sintered at optimal temperature. Mater. Sci. Eng. B Adv. 176(1), 65–67 (2011).
Tian, Y., Gong, Y., Meng, D. & Li, Y. Structure and electrical properties of IrO2-doped 0.5Ba0.7Ca0.3 TiO3–0.5BaTi0.8Zr02O3 ceramics via low-temperature sintering. J. Mater. Sci. 50(18), 6134–6141 (2015).
Wang, P., Li, Y. & Lu, Y. Enhanced piezoelectric properties of (Ba0.85Ca0.15)(Ti0.9Zr0.1)O3 lead-free ceramics by optimizing calcination and sintering temperature. J. Eur. Ceram. Soc. 31(11), 2005–2012 (2011).
Zuo, Q., Luo, L. & Yao, Y. The electrical, upconversion emission, and temperature sensing properties of Er3+/Yb3+-codoped Ba(Zr0.2Ti0.8)O3-(Ba0.7Ca0.3)TiO3 ferroelectric ceramics. J. Alloys Compd. 632(25), 711–716 (2015).
Puli, V. S. et al. Structure, dielectric, ferroelectric, and energy density properties of (1–x) BZT-xBCT ceramic capacitors for energy storage applications. J. Mater. Sci. 48(5), 2151–2157 (2013).
Chen, L., Jia, C. & Wang, K. Spark plasma sintering of high-toughness ZrO2 materials doped with yttrium. Rare Met. 27(5), 479–483 (2008).
Bian, K. et al. Improved sintering activity and piezoelectric properties of PZT ceramics from hydrothermally synthesized powders with Pb excess. J. Mater. Sci. Mater. Electron. 27(8), 8573–8579 (2016).
Tian, Y., Gong, Y., Meng, D. & Cao, S. Structure and electrical properties of Ir4+-doped 0.5Ba0.9Ca0.1TiO3–05BaTi0.88Zr0.12O3–012%La ceramics via a modified Pechini method. Mater. Lett. 153(15), 44–46 (2015).
Nan, B. et al. Direct ink writing of macroporous lead-free piezoelectric Ba0.85Ca0.15Zr0.1Ti0.9O3. J. Am. Ceram. Soc. 102(6), 3191–3203 (2019).
Tian, Y., Gong, Y., Zhang, Z. & Meng, D. Phase evolutions and electric properties of BaTiO3 ceramics by a low-temperature sintering process. J. Mater. Sci. Mater. Electron. 25(12), 5467–5474 (2014).
Tang, X. G., Wang, J., Wang, X. X. & Chan, H. L. W. Effects of grain size on the dielectric properties and tunabilities of sol–gel derived Ba(Zr0.2Ti0.8)O3 ceramics. Solid State Commun. 131(3–4), 163–168 (2004).
Tang, X. G. & Chan, H. L. W. Effect of grain size on the electrical properties of (Ba, Ca)(Zr, Ti)O3 relaxor ferroelectric ceramics. J. Appl. Phys. 97(3), 034109 (2005).
Tian, Y., Gong, Y., Meng, D., Li, Y. & Kuang, B. Dielectric dispersion, diffuse phase transition, and electrical properties of BCT-BZT ceramics sintered at a low-temperature. J. Electron. Mater. 44(8), 2890–2897 (2015).
Uchino, K. & Nomura, S. Critical exponents of the dielectric constants in diffused-phase-transition crystals. Ferroelectrics 44(1), 55–61 (1982).
Lin, J. et al. Effects of compositional changes on up-conversion photoluminescence and electrical properties of lead-free Er-doped K0.5Na0.5NbO3-SrTiO3 transparent ceramics. J. Alloys Compd. 784(5), 60–67 (2019).
Nie, X. et al. Influence of Ca2+ concentration on structure and electrical properties of (Ba1-xCax)(Zr02Ti08)O3 ceramics. Mater. Res. Express 5(3), 036301 (2018).
Li, W., Xu, Z., Chu, R., Fu, P. & Zang, G. Piezoelectric and dielectric properties of (Ba1-xCax)(Ti0.95Zr0.05)O3 lead-free ceramics. J. Am. Ceram. Soc. 93(10), 2942–2944 (2010).
Hunpratub, S., Maensiri, S. & Chindaprasirt, P. Synthesis and characterization of Ba0.85Ca0.15Ti0.9Zr0.1O3 ceramics by hydrothermal method. Ceram. Int. 40(8), 13025–13031 (2014).
Wu, J., Xiao, D., Wu, W., Zhu, J. & Wang, J. Effect of dwell time during sintering on piezoelectric properties of (Ba0.85Ca0.15)(Ti0.90Zr0.10)O3 lead-free ceramics. J. Alloys Compd. 509(41), 359–361 (2011).
Acknowledgement
This work was financially supported by National Natural Science Foundation of China (51972252), and Fundamental Research Funds for the Central Universities (WUT: 2019III029). Xiang Ji was supported to study at the Tokyo Institute of Technology for one year through China Scholarship Council (CSC).
Author information
Authors and Affiliations
Contributions
J.X. and W.C. wrote the main manuscript text and prepared figures. T.H. and S.J. answered the difficulties in the experiment and help complete some tests. S.Z., R.T. and Q.S. provided some funding for our work. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ji, X., Wang, C., Harumoto, T. et al. Structure and electrical properties of BCZT ceramics derived from microwave-assisted sol–gel-hydrothermal synthesized powders. Sci Rep 10, 20352 (2020). https://doi.org/10.1038/s41598-020-73784-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73784-9
This article is cited by
-
Structure and sintering characteristics of rapid synthesizing BCZT powders by microwave assisted sol–gel-hydrothermal method at low temperature
Journal of Materials Science: Materials in Electronics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.