Abstract
The use of microorganisms that allows the recovery of critical high-tech elements such as gallium (Ga) and indium (In) has been considered an excellent eco-strategy. In this perspective, it is relevant to understand the strategies of Ga and In resistant strains to cope with these critical metals. This study aimed to explore the effect of these metals on two Ga/In resistant strains and to scrutinize the biological processes behind the oxidative stress in response to exposure to these critical metals. Two strains of Serratia fonticola, A3242 and B2A1Ga1, with high resistance to Ga and In, were submitted to metal stress and their protein profiles showed an overexpressed Superoxide Dismutase (SOD) in presence of In. Results of inhibitor-protein native gel incubations identified the overexpressed enzyme as a Fe-SOD. Both strains exhibited a huge increase of oxidative stress when exposed to indium, visible by an extreme high amount of reactive oxygen species (ROS) production. The toxicity induced by indium triggered biological mechanisms of stress control namely, the decrease in reduced glutathione/total glutathione levels and an increase in the SOD activity. The effect of gallium in cells was not so boisterous, visible only by the decrease of reduced glutathione levels. Analysis of the cellular metabolic viability revealed that each strain was affected differently by the critical metals, which could be related to the distinct metal uptakes. Strain A3242 accumulated more Ga and In in comparison to strain B2A1Ga1, and showed lower metabolic activity. Understanding the biological response of the two metal resistant strains of S. fonticola to stress induced by Ga and In will tackle the current gap of information related with bacteria-critical metals interactions.
Similar content being viewed by others
Introduction
Indium (In) and gallium (Ga) are metals belonging to group 13 on the periodic table1 that have diverse applications on the aerospace, telecommunication and informatics industries. Gallium is used in a wide variety of products as gallium arsenide (GaAs) or gallium nitride (GaN). These compounds are used in the manufacture of solar cells, LEDs, lasers and in the production of highly specialized circuits that are essential in high-performance computers and cell phones2. The main applications of indium are the production of LCDs and touch screens and it is also used in the manufacture of LEDs and some medical materials3. Face on the economic, industrial and technological importance of both metals and their low abundance comparatively with other elements and metals, Ga and In are included in the critical raw materials list4. With the increase consume of these critical metals, the continuous extraction of these metals from mine ores, secondary mineral deposits or discarded materials is necessary and consequently, the environment is often exposed to different metals resulting in environmental contamination, which disturbs the natural microbial communities of these sites5. However, it is known that several organisms have acquired diverse metal resistance mechanisms such as: (i) change of the metal redox state; (ii) metal cell impermeability; (iii) metals precipitation or ligation to the cell wall, (iv) uptake and intracellular chelation, (v) efflux mechanism enhancing the metal excretion, (vi) secretion of metabolites and (vii) secretion of metal chelating agents (or metabolites) to the environment6. Additionally, when metals enter into the cells, they often lead to an increase in reactive oxygen species (ROS) production. These species are very instable and highly reactive, which result in nucleic acids damage (mutagenic effect), protein damage (protein oxidation with loss of function) and membrane instability that compromise the membrane integrity (with degradation of the cell membrane, lipid peroxidation, and/or inhibition of electron transport chain)7. Therefore, organisms have developed different defence mechanisms to protect cells from these harmful effects. These mechanisms can involve activation of enzymatic antioxidants such as superoxide dismutases (SODs), catalase and peroxidases and non-enzymatic antioxidants as glutathione (GSH) and pigment production8. SOD is considered as first line of defence against oxidative stress by converting O2− into H2O2 and catalase or/and peroxidases complete the detoxification cycle converting H2O2 to O2 and H2O9. In bacteria, SODs are the most important enzymes related to the cellular oxidative stress combat and are often categorized in different families based on its metallic cofactor. The first family has three SOD enzymes: Fe-SOD (SOD with iron as cofactor), Mn-SOD (with manganese) and the cambialistic SOD (which can have iron or manganese as the metallic cofactor). A second family is composed by CuZn-SOD (with copper and zinc) and is mainly found on cytosol of eukaryotic organisms. The last one is Ni-SOD (SOD with nickel) and was described on marine actinomycetes (Actinobacteria) and cyanobacteria10,11,12.
Many reports describe the oxidative stress promoted by heavy metals in bacteria. For example, Rhodobacter capsulatus and Ochrobactrum tritici were described to show an increase in SOD activity when the cells were exposed to tellurite and chromate, respectively13,14. Moreover, a SOD overexpression was also observed with Rhodobacter sphaeroides cells exposed to selenite15 and with Proteus mirabilis exposed to cadmium and lead16.
The presence of antioxidant molecules like glutathione (γ-GluCysGly, reduced form) (GSH) play a significant role in scavenging ROS in living cells. The principal reaction involving GSH is the hydrogen peroxide (H2O2) degradation to water (2GSH + H2O2 → GSSG + 2H2O)17. In bacterial cells, there are some studies relating reduced glutathione levels with resistance of Escherichia coli cells to oxidative stress conditions, such as heavy metals, osmotic stress and some antibiotics18,19. Thus, levels of GSH in cells are used as a biological parameter to evaluate the cellular oxidative stress.
Concerning the bacterial interaction with Ga and In, there is very scarce information. However, Ga seems to enter into the bacterial cells by not completely known transporters. In literature, some works reports that mutants strains (with E. coli and Pseudomonas aeruginosa strains) showed a loss of Ga resistance when genes related to iron (Fe) mechanisms were mutated20,21. Those works lead to the hypothesis that Fe and Ga metabolisms are related, and that Fe-metabolism can affect Ga resistance mechanisms. Another work demonstrated that Ga quenches siderophores, outside the cell, which lead to bacteria starvation and metabolic stress with the absence of Fe inside the cells22. Up to our knowledge, there are no studies that unveil the molecular basis of the resistance mechanisms for indium.
In this work, two Serratia fonticola strains isolated from metal contaminated environments were used. S. fonticola strains are Gram-negative bacteria from Enterobacteriaceae family that can be found in different environments, including drinking water, soil, sewage, humans and animals23. Both strains resistant to the critical metals Ga and In were selected to study the main effects of Ga and In on these resistant strains and to correlate the high resistance to these metals with the activation of biological strategies for the detoxification of the metal, namely the potential mechanisms behind the oxidative stress generated in response to the presence of these critical metals.
Since the literature related to the present subject is very scarce, this study will help to understand the effect of these two metals in bacterial cells and how bacterial strains are able to survive in presence of critical metals.
Results
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The MIC and MBC values obtained for both metals, in liquid assays, are shown in Table 1. While the MIC value for In was 0.75 mM for both strains, the MIC values for Ga were 1.5 mM and 2 mM for strains A3242 and B2A1Ga1, respectively. The MBC for In was only quantified for strain A3242, because compared to other situations, the MBC values are higher than the maximum concentration tested, 2 mM Ga and 1 mM In.
Analysis of protein expression
The protein expression profile of both strains grown in presence of metals was evaluated and compared with a control situation (grown in absence of metals). Figure 1 shows that both strains, when exposed to In, overexpressed a protein with a molecular weight of approximately 25 kDa (protein band marked with an arrow). These protein bands were cut and identified by mass spectrometry with Mascot server, using the available UniProt database within the taxonomic genera Serratia, as belonging to iron/manganese superoxide dismutase family.
SDS-PAGE (12%) of the protein profile of bacteria grown without and with metals (0.4 mM Ga and 0.2 mM In): (A) M—low molecular weight protein marker (NZYTech), 1—A3242 control, 2—A3242 with Ga, 3—A3242 with In; (B) M—NZYColour protein marker II (NZYTech), 1—B2A1Ga1 control, 2—B2A1Ga1 with Ga, 3—B2A1Ga1 with In. The overexpressed protein band was marked with arrow.
Metal quantification
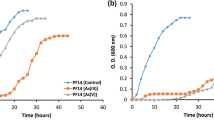
Growth curves of the strains in absence and presence of Ga and In were performed to show the effect of metals on bacterial growth and to select the time points to collect samples with cellular growth enough for metal quantification assays (Fig. 2). Indium had a more drastic effect in the normal growth of both strains when compared to Ga and this effect was more visible for strain A3242. Based on growth curves, the cells for metal quantification analyses were taken at two different times, 6 h of growth, corresponding to the end of exponential growth phase and 24 h, corresponding to the late stationary growth phase.
The In and Ga uptake by the strains, analysed by ICP-MS, showed that strains have different levels of metal accumulation, as shown in Fig. 3. However, both strains showed higher accumulation of metals at 6 h than at 24 h of bacterial growth.
The strain A3242 showed the highest Ga levels in cells with approximately 0.64 and 0.30 µg Ga/mg protein at 6 h and 24 h, respectively, comparing with strain B2A1Ga1 that accumulated approximately 0.13 and 0.10 µg Ga/mg protein at 6 h and 24 h, respectively. In the case of In, strain A3242 also showed higher accumulation at 6 h (approximately 31.31 µg In/mg) than strain B2A1Ga1 (19.53 µg In/mg protein). At 24 h of bacterial growth, strains A3242 and B2A1Ga1 only accumulated 16.40 and 3.35 µg In/mg protein, respectively. Results suggest that metals are pumping out from the cells at the late stationary phase. Quantification of Ga in the growth medium of the best metal accumulator, strain A3232 showed values of 17,534.3 ± 253 ppm, 15,803.8 ± 8.6 ppm and 17,452 ± 35.9 ppm at 0 h, 6 h and 24 h, respectively. The In values determined in the growth medium of the strain were 7961.8 ± 65 ppm, 6395.1 ± 17.2 ppm and 6947 ± 56.1 ppm, respectively. Thus, the increase of metals in the growth medium at 24 h confirmed the release of Ga and In from cells.
Cellular metabolic activity
The cellular metabolic activity was studied in both strains using the MTT assay and the results are shown in Fig. 4. Strain A3242 revealed a significant decrease of the cellular metabolic activity in the presence of In, showing activity values of 5.6 and 4.2 fold lower than the control, for samples taken at 6 h and 24 h of incubation, respectively. In the case of strain B2A1Ga1, Ga or In did not affect significantly its metabolic activity.
MTT assays of strains A3242 (A) and B2A1Ga1 (B). Data shown are the mean values (± standard deviations) obtained from three independent experiments. ***Significantly different from the value of Control (without metal), p < 0.001, respectively. Relative Optical Density Units (RODU) means the ratio between the OD550 (absorbance at 550 nm) of the sample (test) and the OD550 of the control experiment.
ROS quantification
The oxidative stress induced by Ga and In was studied through the quantification of the intracellular ROS concentration in cells exposed to those metals and compared with control (growth without metals).
In Fig. 5A,B, it is possible to observe that both strains exhibited a significantly higher production of ROS in the presence of In than in the control situation, but did not show more production of ROS with Ga. The strain A3242 showed the highest values of ratio with 3.1, 20.3 and 28.3 fold higher for 0.1 mM, 0.2 mM and 0.4 mM of In, respectively. Strain B2A1Ga1 showed ratio values of 1.4 and 5.9 fold higher than control for 0.2 mM and 0.4 mM of In, respectively.
Ratio values of the relative fluorescence units (RFU of the test assay/RFU of the control assay) obtained for strains A3242 (A) and B2A1Ga1 (B). Data shown are the mean values (± standard deviations) obtained from three independent experiments. ***Significantly different from the value of control (without metal), p < 0.001.
Reduced glutathione quantification
Reduced glutathione was also quantified to confirm the potential effect of Ga and In in the oxidative stress induction. Reduced glutathione is considered an antioxidant thiol and lower concentrations of reduced glutathione (in relation with the total intracellular glutathione) mean higher oxidative stress.
The Fig. 6 shows that both strains with In showed a significantly lower ratio GSH/total glutathione than the control with a decrease of 3.2 and 2.8 fold for A3242 and B2A1Ga1, respectively). In cells exposure to Ga, this ratio for both strains was also significantly lower than in control. However, the decrease of ratio GSH/total glutathione was just 1.3 and 1.2 fold of the control for A3242 and B2A1Ga1, respectively.
Ratio values between the reduced glutathione and the total intracellular glutathione of strains A3242 (A) and B2A1Ga1 (B). Data shown are the mean values (± standard deviations) obtained from two independent experiments. ***Significantly different from the value of control (without metal), p < 0.001.
SOD activity in solution
The analysis of SOD activity was performed for both strains since they showed the overexpression of a SOD enzyme in the presence of In (visible by SDS-PAGE). Figure 7 shows that both strains exhibited significantly higher SOD activity in the samples with In than in the samples with Ga or without metal. Indium exposure induced an increase of approximately twofold of SOD activity compared to the control situation without metal.
SOD activity (U/mg protein) of strains A3242 (A) and B2A1Ga1 (B), in absence and presence of the critical metals, Data shown are the mean values (± standard deviations) obtained from two independent experiments. *,**Significantly different from the value of control (without metal), p < 0.05 and p < 0.01, respectively.
SOD activity staining
Soluble protein samples of both strains, obtained from the growth in the presence and absence of In, were run in NBT-PAGE electrophoresis. A sample from Escherichia coli BL21 was used as a reference. To have a correct identification of the SOD present in the samples, different treatments of the gels were performed. Therefore, gels were incubated with two different inhibitor solutions: KCN and H2O2.
In absence of inhibitors (Fig. 8A), protein samples of both strains from growths in the presence of In showed an additional SOD band compared to the control. When incubated with 10 mM H2O2 (Fig. 8B), an inhibitor of Fe-SOD and Cu/Zn-SOD, the lower band disappeared in In samples and in the reference strain (E. coli BL21). However, with 10 mM KCN (Fig. 8C), an inhibitor of Cu/Zn-SOD, the SOD bands were similar to the control gel (enzymatic reaction without inhibitor), being visible the additional lower SOD band in samples of In. These results suggest that Serratia strains do not have Cu/Zn-SOD and that the induced SOD by In is a Fe-SOD.
Activity staining of the protein samples electrophoresed on three independent 10% nondenatured polyacrylamide gels: (A) enzymatic reaction in absence of inhibitors; (B) enzyme incubated with 5 mM H2O2; (C) enzyme incubated with 5 mM KCN. Arrows indicated the additional SOD band. Original gels shown in Fig. S1.
Discussion
This work is focused on two Serratia strains, named A3242 and B2A1Ga1, isolated from the metal-contaminated mines of Urgeiriça and Panasqueira. Urgeiriça environment is naturally contaminated with uranium24 and Panasqueira with tungsten but other metals are also present in small or trace quantities25. Despite these sites are not reported as gallium or indium contaminated, both isolates showed extraordinary resistance to both critical metals, exhibiting high MIC values (0.75 mM for In and 1.5 mM or 2 mM for Ga) while a large number of isolates, including other tested Serratia strains, showed MICs of 1 mM for Ga and 0.25 mM for In as the highest values. The natural presence of metals in these environments may pressure the autochthonous bacterial communities, selecting the most resistant microorganisms to metals in general.
There are very few works exploring Bacteria-Ga/In interactions, therefore, this work aimed to understand the biological mechanisms behind oxidative stress generated in response to exposure to critical metals and its control by the cells. In this sense, these two very resistant strains were studied concerning the differential protein expression (under or overexpression) when grown with those critical metals, visible on SDS-PAGE gels. Only In induced detectable overexpression of proteins, identified as SOD enzymes. These enzymes are usually related to the control of cellular oxidative stress9.
The SOD activity detected in the soluble protein fractions was used to identify the additional SOD induced by In. It is known that KCN does not inhibit both Fe-SOD and Mn-SOD (inhibits CuZn-SOD) and H2O2 does not inhibit Mn-SOD but inhibits Fe-SOD and CuZn-SOD26. In this work, the inhibitory reactions were compared enabling the identification of the metallic cofactor present in the additional SOD enzyme. It was inhibited by H2O2 but not by KCN concluding that this enzyme was a Fe-SOD. Considering all the results observed with both SOD activity assays, it is possible to conclude that both strains have higher SOD activities when exposed to In. Previous work, relating the genome of Serratia sp. LCN16 strain with the high tolerance to oxidative stress predicted three genes, encoding three SOD-enzymes: two Fe/Mn-SOD (sod2) and one Cu/Zn-SOD (sod1)27. Up to our knowledge, currently there is no work relating the induction of SODs in Serratia strains with the presence of Ga and In. Nevertheless, for some strains, it was already described an overexpression of one specific SOD in the presence of heavy metals, such as overexpression of Mn-SOD with cadmium and lead in Proteus mirabilis, and overexpression of Fe-SOD with chromate in Ralstonia metallidurans16,28. A recent study reported a gradual and significant increase of the SOD activity in presence of antimony concentrations up to 10 mM in a strain of Serratia marcescens isolated from roots of the Hedysarum pallidum plant29.
It is well reported that metals are often responsible for oxidative stress in the cell (one cellular consequence from metal stress), which can affect the bacterial growth and even result in cell death30. Oxidative stress can be analysed using different approaches, such as ROS quantification using probes, protein damage quantification (protein carbonyl content), lipid peroxidation evaluation (malondialdehyde content), DNA damage visualization, measurement of non-enzymatic antioxidants (e.g. reduced glutathione) and assessment of enzymatic antioxidants (e.g. superoxide dismutase, catalase activities)31. In this work, the ROS levels quantified for both selected strains revealed that, in general, the strains showed a high ROS concentration when incubated with high concentrations of In. This is the first study to clearly show the effect of In salts in the increase of ROS production in bacterial cells. Previous works only showed the consequence of Indium Tin Oxide (ITO) nanoparticles exposure in the increase of the intracellular ROS levels on human cells32,33. The strains did not show a high production of ROS when exposed to high Ga concentrations, suggesting that Ga (at tested concentrations) did not induce oxidative stress in those bacteria. These results are according to the analysis of the SDS-PAGE profile. In the presence of In, both bacteria showed high oxidative stress, which resulted in high SOD activity to detoxify the cellular ROS.
The oxidative stress induced by the target critical metals was also confirmed by quantification of the reduced glutathione levels. This antioxidant exists in the reduced form in low quantities inside the cells when the cells are under oxidative stress conditions19. Both strains showed lower ratios (reduced glutathione/total glutathione) when subjected to the critical metals. The ratio decrease was especially relevant in the presence of In. This last result is in agreement with the ROS levels detected in cells, confirming that in S. fonticola, In induces high oxidative stress.
The effect of Ga and In on cellular metabolic activity was assessed by MTT assays. The decrease of activity in cells is considered a good indicator of cell redox activity34. The strains were affected by the exposition to both critical metals, but indium exhibited the most drastic toxic effect. Analysing the results of cellular viability, the strains, despite belonging to the same species, did not show an identical profile when exposed to the critical metals.
The two Serratia strains A3242 and B2A1Ga1 come from different environments and this fact can justify the notable difference in the cellular viability of these strains when grown with In. The differences between strains could also be related to different metal uptakes. The strain able to accumulate more metal showed the strongest decrease in metabolic activity. It is known that high amounts of metal inside the cells might result in many toxic effects in cells, resulting in lower cellular viability13. Strains showed the trend to pump metal out of cells at the late stationary phase (24 h of incubation) of growth. Metal efflux mechanisms (present or inducible) in bacteria decrease the cellular damage resulting from metal accumulation and are often responsible for bacterial metal resistance30,35,36. There is no description of mechanisms to pump-out In and Ga from the cells. However, considering the effect of both metals in the cellular viability, it is possible that bacteria pump-out metal in the stationary phase to limit the cellular damage (DNA, proteins, lipids). For instance, studies with E. coli and Bacillus subtilis showed that genes coding for putative manganese efflux pumps are upregulated when the bacteria are exposed to manganese37,38.
In conclusion, the critical metals focused on this work, exhibited different impact in two S. fonticola strains. Both strains when exposed to Ga just showed a significantly decrease of the ratio GSH/total glutathione, while they exhibited an increase of oxidative stress when exposed to In, with activation of biological mechanisms to control that stress, such as reduced glutathione and SOD enzymes. These strains were even able to activate an additional SOD enzyme (Fe-SOD) as a response to the toxicity induced by In. Understanding the strategies of metal resistant bacteria to cope with critical metals is important to enable and promote the use of bacteria in innovative and sustainable processes for metal recovery.
Methods
Bacterial strains, media and growth conditions
S. fonticola A3242 (isolated from Urgeiriça mine) and S. fonticola B2A1Ga1 (isolated from Panasqueira mine) were grown in Reasoner's 2A broth medium (R2Ab), containing per liter: 0.5 g of yeast extract, 0.5 g of proteose peptone, 0.5 g of casein, 0.5 g of glucose, 0.5 g of soluble starch, 0.3 g of K2HPO4, 0.024 g of MgSO4 and 0.3 g of sodium pyruvate. Bacterial growth was evaluated after incubation at 30 ºC, measuring the optical density at 600 nm (OD600). Metal stock solutions were prepared in a concentration of 0.5 M indium(III) chloride (InCl3) (Acros Organics) and 0.2 M gallium(III) nitrate (GaN3O9) (Alfa Aesar) and were sterilized by filtration.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) assays
The MIC of Ga and In were evaluated using the standard broth microdilution method in R2Ab39. Each assay was repeated in triplicate.
Additionally, the assays with metal concentrations equal and above the MIC values obtained were used to determinate the MBC. Therefore, bacterial suspensions from the wells with Ga or In concentrations that did not show bacterial growth were plated onto R2A solid medium. Bacterial growths were analysed after 48 h of incubation at 30 ºC.
Analysis of protein expression
The bacterial resistance mechanisms to metals might be related to differential expression (under or overexpression) of specific proteins. Therefore, the protein expression profiles of cells grown in the absence (control) or presence of critical metals (0.1 mM In or 0.2 mM Ga) were evaluated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE). Firstly, the cells from 3 days of growth were centrifuged and washed twice with Phosphate Buffer Saline solution (PBS—8 g/l NaCl, 0.2 g/l KCl, 1.44 g/l Na2HPO4, 0.24 g/l KH2PO4, pH 7.4). The pellet was resuspended in 300 µl of PBS and disrupted by four cycles of 30 s pulse sonication (Sonics & Materials Inc. Danbury, Connecticut U.S.A.) at 60 A. The samples were centrifuged at 13,000 rpm (rotation per minute) for 15 min and the protein content in each fraction was quantified by Bradford method40. The search for a differential protein expression profile was evaluated by 0.1% sodium dodecyl sulfate (SDS)—12% polyacrylamide gel electrophoresis (PAGE), with a Coomassie Blue staining. The protein bands overexpressed in presence of the metals were identified by peptide mass spectrometry with Mascot server (Maldi-TOF, IPATIMUP, Porto).
Metal quantification
Strains were grown in R2Ab at 30 ºC with 140 rpm spiked with 0.2 mM Ga and 0.1 mM In. Samples of bacterial growth were collected at 6 hand 24 h.
The samples were centrifuged three times at 4000 rpm for 20 min at 4 ºC, the cellular pellets were washed twice with cold PBS solution and the final bacterial pellets were lysed with an acid treatment (5% HNO3), heated at 50 ºC for 1 h and then centrifuged at 13,000 rpm for 10 min. The intracellular supernatants and the medium from bacterial growth at a specific time of sampling (0 h, 6 h and 24 h) were diluted for the metal quantification by Inductively Coupled Plasma Mass Spectrometry (ICP-MS)41. Pellets were neutralized with NaOH 0.5 M and then used to quantify the total protein by Bradford method40.
Test of cellular metabolic activity
The cellular metabolic activity was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay (MTT assay), according to the protocol of Wang and colaborators42 modified as described. Briefly, 1 ml of cellular growth, taken at the incubation times previously referred, were centrifuged, washed twice with R2Ab and the pellets were resuspended in 1 ml of medium. The cell suspensions were diluted with R2Ab to an OD600 of 0.2 and mixed 10:1 with MTT stock solution (5 g/ml). The mixtures were incubated with the cap tube open at 30 ºC for 1 h. After the incubation time, the mixtures were centrifuged at 10,000 g for 2 min and the pellets dissolved in 2.5 ml of dimethyl sulfoxide (DMSO). The samples were incubated 1 h at room temperature before quantified spectrophotometrically at 550 nm.
ROS quantification
ROS concentration in the cells was quantified using the 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) method43. The protocol followed was based on the used method in a previous work14 and was optimized to the current assays with Ga and In. Strains grown in R2Ab, were exposed to different metal concentrations when growth reached OD600 of 0.2–0.3, and 2 h later, exposed to 25 μM of H2DCFDA for 1 h. The cells were centrifuged, washed twice with PBS and the pellets were resuspended in 1 ml PBS. The fluorescence (λem = 527 nm and λex = 495 nm) and OD600 were read hourly during 5 h. ROS are determined as the Relative Fluorescence Units (RFU), obtained from the fraction between the fluorescence levels measured at λem = 527 nm and λex = 495 nm and the absorbance measured at 600 nm. The values of ratio of ROS are shown as the fraction of RFU value determined in the metal assay and the RFU value in the control situation (without metal).
Reduced glutathione quantification
The glutathione levels in solution was quantified using the Ellman’s reagent, also known as 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) that reacts with free sulfhydryl groups in solution, using a previously described protocol18.
The cells were grown overnight (16 h) in R2Ab with different metal concentrations. After centrifugation, cells were washed twice with ice-cold PBS solution and the pellets were resuspended in 400 µl of 50 mM sodium phosphate, pH 7.4, (28 ml of 0.2 M NaH2PO4, 72 ml of 0.2 M Na2HPO4 and distilled water to a final volume of 400 ml) with proteases inhibitors (1 protease inhibitor cocktail tablet, EDTA-free, per 100 ml of buffer). Cells kept on ice were disrupted by four cycles of sonication (Sonics & Materials Inc. Danbury, Connecticut USA) at 60 A, with pulses of 30 s. Then, the samples were centrifuged for 1 h at 13,000 rpm to obtain soluble protein in the clear supernatant.
After protein quantification40, a stock for each sample was prepared with a protein concentration of 1 mg/ml (dilution prepared with 50 mM sodium phosphate buffer, pH 7.4). The assays were prepared to calculate the reduced glutathione and the total glutathione. For the first quantification, mixtures comprising sample, 6 mM DTNB and ultrapure water, 1:1:8.1, respectively, were incubated at room temperature for 6 min and the absorbance was measured at 412 nm. Total glutathione quantification assays were prepared mixing 50 µl of sample, 0.15 ml of ultrapure water, 0.7 ml of 0.3 mM NADPH, 0.1 ml of 6 mM DTNB and 10 µl of glutathione reductase solution (50 U/ml) and 6 min later the absorbance at 412 nm was measured.
SOD activity in solution
Nitro Blue Tetrazolium (NBT) quantification method44 was used for quantification of SOD activity in solution. In samples with SOD, the superoxide anion (O2−) is converted to H2O2 and the reduction of NBT does not occur. Therefore, it is defined that 1 unit (U) of SOD corresponds to a decline of 50% formation the NBT photoreducted product45.
In this assay, cells were grown and treated as described previously for glutathione quantification experiments. The test reactions were composed by 30 µM NBT, 5 mM methionine and 26.6 µM riboflavin, all solutions prepared in SPB. For each sample, three protein concentrations were tested (10 µg, 20 µg and 30 µg protein per 1 ml of experiment). All the mixtures were subjected to illumination for 10 min with 15 W fluorescent light. After this illumination, the absorbances were read at 560 nm.
SOD activity staining
SOD activity was also analysed by NBT–10% PAGE, with incubations in NBT-Riboflavin solutions14. In this assay, the cells were grown and treated as described previously for glutathione quantification experiments and analysis of SOD activity in solution. The electrophoresis was run with 18 µg of protein (each sample) at 120 V for 75 min at 4º C. The staining of the gel was based on two incubations with shaking for 20 min each, first with a staining solution of 10% riboflavin and 25% NBT and then, with a 0.1% TEMED solution. The identification of the SOD classes present in the samples was performed by adding different inhibitors solutions: 5 mM potassium cyanide solution (KCN) and 5 mM hydrogen peroxide solution (H2O2)14.
Statistical analysis
Each result is indicated as the mean value of two or three independent experiments (number of independent experiments is indicated in the caption of each figure) ± the standard derivation. The statistical analysis of all results was performed using GraphPad Prism version 5.00 for Windows46, using One-way ANOVA followed by Tukey’s multiple comparisons test, except for the analysis of metal quantification (accumulation) and the cellular viability results that used Two-way ANOVA followed by Bonferroni’s multiple comparisons test.
References
Chang, R. & Goldsby, K. A. Chemistry. (McGraw-Hill Education, 2010).
Foley, N. K., Jaskula, B. W., Kimball, B. E. & Schulte, R. F. Professional Paper 1802 in Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply. https://doi.org/10.3133/pp1802H (U.S. Geological Survey, 2017).
Hines, C. J., Roberts, J. L., Andrews, R. N., Jackson, M. V. & Deddens, J. A. Use of and occupational exposure to indium in the United States. J. Occup. Environ. Hyg. 10, 723–733 (2013).
Commission, E. Study on the review of the list of critical raw materials—Critical raw materials factsheets. Eur. Commiss. https://doi.org/10.2873/876644 (2017).
Teitzel, G. M. & Parsek, M. R. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl. Environ. Microbiol. 69, 2313–2320 (2003).
Srivastava, P. & Kowshik, M. Mechanisms of metal resistance and homeostasis in Haloarchaea. Archaea 2013, 732864 (2013).
Wang, L., Hu, C. & Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 12, 1227–1249 (2017).
Lushchak, V. I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 153, 175–190 (2011).
Fridovich, I. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? in Annals of the New York Academy of Sciences, Vol. 893, 13–18 (New York Academy of Sciences, 2006).
Mccord, J. M. & Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244, 6049–6055 (1969).
Roberts, B. & Hirst, R. Identification and characterisation of a superoxide dismutase and catalase from Mycobacterium ulcerans. J. Med. Microbiol. 45, 383–387 (1996).
Scheer, M. et al. BRENDA, the enzyme information system in 2011. Nucleic Acids Res. 39, D670–D676 (2011).
Borsetti, F. et al. Tellurite effects on Rhodobacter capsulatus cell viability and superoxide dismutase activity under oxidative stress conditions. Res. Microbiol. 156, 807–813 (2005).
Branco, R. & Morais, P. V. Two superoxide dismutases from Tn Otchr are involved in detoxification of reactive oxygen species induced by chromate. BMC Microbiol. 16 (2016).
Bebien, M., Chauvin, J. P., Adriano, J. M., Grosse, S. & Verméglio, A. Effect of selenite on growth and protein synthesis in the phototrophic bacterium Rhodobacter sphaeroides. Appl. Environ. Microbiol. 67, 4440–4447 (2001).
Eickhoff, J., Potts, E., Valtos, J. & Niederhoffer, E. C. Heavy metal effects on Proteus mirabilis superoxide dismutase production. FEMS Microbiol. Lett. 132, 271–276 (1995).
Forman, H. J., Zhang, H. & Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 30, 1–12 (2009).
Li, Y., Hugenholtz, J., Abee, T. & Molenaar, D. Glutathione protects Lactococcus lactis against oxidative stress. Appl. Environ. Microbiol. 69, 5739–5745 (2003).
Smirnova, G., Muzyka, N. & Oktyabrsky, O. Transmembrane glutathione cycling in growing Escherichia coli cells. Microbiol. Res. 167, 166–172 (2012).
García-Contreras, R. et al. Gallium induces the production of virulence factors in Pseudomonas aeruginosa. Pathog. Dis. 70, 95–98 (2013).
Gugala, N., Chatfield-Reed, K., Turner, R. J. & Chua, G. Using a chemical genetic screen to enhance our understanding of the antimicrobial properties of gallium against Escherichia coli. Genes (Basel). 10, 34 (2019).
Ross-Gillespie, A., Weigert, M., Brown, S. P. & Kümmerli, R. Gallium-mediated siderophore quenching as an evolutionarily robust antibacterial treatment. Evol. Med. Public Health 2014, 18–29 (2014).
Ruan, J. H. et al. Rapid detection of Serratia fonticola by TaqMan quantitative real-time PCR using primers targeting the gyrB gene. Curr. Microbiol. 74, 1343–1348 (2017).
Sousa, T., Chung, A. P., Pereira, A., Piedade, A. P. & Morais, P. V. Aerobic uranium immobilization by Rhodanobacter A2–61 through formation of intracellular uranium-phosphate complexes. Metallomics 5, 390–397 (2013).
Chung, A. P. et al. Tailings microbial community profile and prediction of its functionality in basins of tungsten mine. Sci. Rep. 9, 19596 (2019).
Ma, X., Deng, D. & Chen, W. Inhibitors and activators of SOD, GSH‐Px, and CAT, enzyme inhibitors and activators. in Enzyme Inhibitors and Activators 207–224. https://doi.org/10.5772/65936 (InTech, 2017).
Vicente, C. S. L. et al. The genome and genetics of a high oxidative stress tolerant Serratia sp. LCN16 isolated from the plant parasitic nematode Bursaphelenchus xylophilus. BMC Genomics 17, 301 (2016).
Roux, M. & Covés, J. The iron-containing superoxide dismutase of Ralstonia metallidurans CH34. FEMS Microbiol. Lett. 210, 129–133 (2002).
Laouar, M. K. et al. Antioxidative enzyme responses to antimony stress of Serratia marcescens—An endophytic bacteria of Hedysarum pallidum roots. Polish J. Environ. Stud. 29, 141–152 (2020).
Chandrangsu, P., Rensing, C. & Helmann, J. D. Metal homeostasis and resistance in bacteria. Nat. Rev. Microbiol. 15, 338–350 (2017).
Katerji, M., Filippova, M. & Duerksen-Hughes, P. Approaches and methods to measure oxidative stress in clinical samples: Research applications in the cancer field. Oxid. Med. Cell. Longev. 2019, 1–29 (2019).
Tabei, Y. et al. In vitro evaluation of the cellular effect of indium tin oxide nanoparticles using the human lung adenocarcinoma A549 cells. Metallomics 7, 816–827 (2015).
Tabei, Y. et al. Intracellular accumulation of indium ions released from nanoparticles induces oxidative stress, proinflammatory response and DNA damage. J. Biochem. 159, 225–237 (2016).
Almutary, A. & Sanderson, B. J. S. The MTT and crystal violet assays: Potential confounders in nanoparticle toxicity testing. Int. J. Toxicol. 35, 454–462 (2016).
Branco, R. et al. The chromate-inducible chrBACF operon from the transposable element TnOtChr confers resistance to chromium(VI) and superoxide. J. Bacteriol. 190, 6996–7003 (2008).
Sousa, T., Branco, R., Piedade, A. P. & Morais, P. V. Hyper accumulation of arsenic in mutants of Ochrobactrum tritici silenced for arsenite efflux pumps. PLoS ONE 10, e0131317 (2015).
Waters, L. S., Sandoval, M. & Storz, G. The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis. J. Bacteriol. 193, 5887–5897 (2011).
Huang, X., Shin, J. H., Pinochet-Barros, A., Su, T. T. & Helmann, J. D. Bacillus subtilis MntR coordinates the transcriptional regulation of manganese uptake and efflux systems. Mol. Microbiol. 103, 253–268 (2017).
Miller, R. A. et al. Standardization of a broth microdilution susceptibility testing method to determine minimum inhibitory concentrations of aquatic bacteria. Dis. Aquat. Organ. 64, 211–222 (2005).
Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Coimbra, C., Branco, R. & Morais, P. V. Efficient bioaccumulation of tungsten by Escherichia coli cells expressing the Sulfitobacter dubius TupBCA system. Syst. Appl. Microbiol. 42, 126001 (2019).
Wang, H., Cheng, H., Wang, F., Wei, D. & Wang, X. An improved 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction assay for evaluating the viability of Escherichia coli cells. J. Microbiol. Methods 82, 330–333 (2010).
Oparka, M. et al. Quantifying ROS levels using CM-H2DCFDA and HyPer. Methods 109, 3–11 (2016).
Tan, B. H., Leow, T. C., Foo, H. L. & Rahim, R. A. Molecular characterization of a recombinant manganese superoxide dismutase from Lactococcus lactis M4. Biomed. Res. Int. 2014, 469298 (2014).
Gonzalez, P. J., Lozano, M., Lascano, H. R., Lagares, A. & Melchiorre, M. N. Increased oxidative stress tolerance by superoxide dismutase overexpression in Mesorhizobium loti. https://doi.org/10.1101/363556. bioRxiv:363556 (2018).
GraphPad Software. GraphPad Prism version 7.00 for Windows. (2007).
Acknowledgements
This study was supported by the project PTDC/CTA-AMB/31820/2017 funded by Fundação para a Ciência e Tecnologia (FCT) and Programa Operacional da Região Centro and by the project ERA-MIN/0002/2015. The research was also sponsored by FEDER funds through the program COMPETE—Programa Operacional Factores de Competitividade—and by national funds through FCT (UIDB/00285/2020).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: R.B. Performed the experiments: J.B.C. Analyzed the data: R.B. and J.B.C. Contributed reagents/materials/analysis tools: R.B. and P.V.M. Wrote the manuscript: J.B.C. and R.B. Revised the manuscript: R.B. and P.V.M. Approved the manuscript: all authors, J.B.C., P.V.M. and R.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Caldeira, J.B., Morais, P.V. & Branco, R. Exploiting the biological response of two Serratia fonticola strains to the critical metals, gallium and indium. Sci Rep 10, 20348 (2020). https://doi.org/10.1038/s41598-020-77447-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-77447-7
This article is cited by
-
Relevance of FeoAB system in Rhodanobacter sp. B2A1Ga4 resistance to heavy metals, aluminium, gallium, and indium
Applied Microbiology and Biotechnology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.