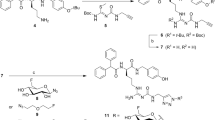
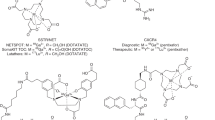
Abstract
Here, we describe an extension of our silicon fluoride acceptor (SiFA) protocol for 18F-labeling of peptides that addresses challenges associated with preparing a clinical-grade (Tyr3)-octreotate (TATE) tracer for diagnosis of neuroendocrine tumors (NETs). After several iterations of protocol optimization (e.g., finding the optimal pH at which the isotopic exchange (IE) reaction produces high radiochemical yields (RCYs)), the SiFA technology achieved clinical applicability, as showcased by radiosynthesis of [18F]SiFAlin-TATE ([18F]SiTATE), the first SiFA peptide used in the clinical diagnosis of NETs. The TATE peptide binds to somatostatin receptors associated with NETs. Radiolabeled TATE derivatives are routinely applied in clinical oncological PET imaging. The (SiFA) 18F-labeling technology is based on the IE of a 19F atom for a radioactive 18F atom, a highly efficient labeling reaction under mild conditions. The 19F is part of a biomolecule bearing the SiFA building block, composed of a central silicon (Si) atom, a 19F atom connected to the Si atom, and two Si-bound tert-butyl groups. The IE proceeds through a penta-coordinate bipyramidal intermediate, followed by elimination of non-radioactive 19F, yielding the labeled compound in high RCYs at room temperature (22 °C). The simplicity and lack of side-product formation of this approach enable a one-step, kit-like preparation of structurally complex and unprotected radiopharmaceuticals. Compounds such as peptides used for tumor imaging in nuclear medicine can be 18F-labeled without the need for complex purification protocols. [18F]SiTATE can be synthesized within 30 min in preparative RCYs of 42%, radiochemical purity of >97% and high molar activity of 60 GBq/µmol.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Figures 3 and 5 show example HPLC chromatograms. These data are shown for the first time in these figures. Figure 4 includes information about the yields of the reactions, and the Anticipated results section contains their analytical data; these have been described in previous work, except for compound 7, which is included in this article.
References
Olberg, D. E. & Hjelstuen, O. K. Labeling strategies of peptides with 18F for positron emission tomography. Curr. Top. Med. Chem. 10, 1669–1679 (2010).
Okarvi, S. Recent progress in fluorine-18 labelled peptide radiopharmaceuticals. Eur. J. Nuc. Med. 28, 929–938 (2001).
Cai, L., Lu, S. & Pike, V. W. Chemistry with [18F]fluoride ion. Eur. J. Org. Chem. 2008, 2853–2873 (2008).
Shukla, A. K. & Kumar, U. Positron emission tomography: an overview. J. Med. Phys. 31, 13–21 (2006).
Purser, S., Moore, P. R., Swallow, S. & Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 37, 320–330 (2008).
Banerjee, S. R. & Pomper, M. G. Clinical applications of gallium-68. Appl. Radiat. Isot. 76, 2–13 (2013).
Smith, D. L., Breeman, W. A. P. & Sims-Mourtada, J. The untapped potential of gallium 68-PET: the next wave of 68Ga-agents. Appl. Radiat. Isot. 76, 14–23 (2013).
Al-Nahhas, A. et al. Gallium-68 PET: a new frontier in receptor cancer imaging. Anticancer Res. 27, 4087–4094 (2007).
Sanchez-Crespo, A. Comparison of gallium-68 and fluorine-18 imaging characteristics in positron emission tomography. Appl. Radiat. Isot. 76, 55–62 (2013).
Decristoforo, C. Gallium-68—a new opportunity for PET available from a long shelflife generator—automation and applications. Curr. Radiopharm. 5, 212–220 (2012).
Alves, F. et al. Production of copper-64 and gallium-68 with a medical cyclotron using liquid targets. Mod. Phys. Lett. A 32, 1740013 (2017).
Riga, S. et al. Production of Ga-68 with a General Electric PETtrace cyclotron by liquid target. Physica Med. 55, 116–126 (2018).
Pandey, M. K., Byrne, J. F., Jiang, H., Packard, A. B. & DeGrado, T. R. Cyclotron production of (68)Ga via the (68)Zn(p,n)(68)Ga reaction in aqueous solution. Am. J. Nucl. Med. Mol. Imaging 4, 303–310 (2014).
Nelson, B. J. B. et al. Taking cyclotron 68Ga production to the next level: expeditious solid target production of 68Ga for preparation of radiotracers. Nucl. Med. Biol. 80-81, 24–31 (2020).
Ilhan, H. et al. Biodistribution and first clinical results of 18F-SiFAlin-TATE PET—a novel 18F-labeled somatostatin analog for imaging of neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 47, 870–880 (2019).
Hope, T. A., Calais, J., Zhang, L., Dieckmann, W. & Millo, C. 111In-pentetreotide scintigraphy versus 68Ga-DOTATATE PET: impact on Krenning scores and effect of tumor burden. J. Nucl. Med. 60, 1266–1269 (2019).
Hofman, M. S., Lau, W. F. E. & Hicks, R. J. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. RadioGraphics 35, 500–516 (2015).
Deppen, S. A. et al. Safety and efficacy of 68Ga-DOTATATE PET/CT for diagnosis, staging, and treatment management of neuroendocrine tumors. J. Nucl. Med. 57, 708–714 (2016).
Waldmann, C. M., Stuparu, A. D., van Dam, R. M. & Slavik, R. The search for an alternative to [(68)Ga]Ga-DOTA-TATE in neuroendocrine tumor theranostics: current state of (18)F-labeled somatostatin analog development. Theranostics 9, 1336–1347 (2019).
Bozkurt, M. F. et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F–DOPA. Eur. J. Nucl. Med. Mol. Imaging 44, 1588–1601 (2017).
Dasari, A. et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 3, 1335–1342 (2017).
Ilhan, H. et al. First-in-human 18F-SiFAlin-TATE PET/CT for NET imaging and theranostics. Eur. J. Nucl. Med. Mol. Imaging 46, 2400–2401 (2019).
Schirrmacher, R. et al. Small prosthetic groups in 18F-radiochemistry: useful auxiliaries for the design of 18F-PET tracers. Semin. Nucl. Med. 47, 474–492 (2017).
Schirrmacher, R. et al. 18F-Labeling of peptides by means of an organosilicon-based fluoride acceptor. Angew. Chem., Int. Ed. 45, 6047–6050 (2006).
Wängler, C. et al. Silicon-[18F]fluorine radiochemistry: basics, applications and challenges. Appl. Sci. 2, 277–302 (2012).
Bernard-Gauthier, V. et al. From unorthodox to established: the current status of 18F-trifluoroborate- and 18F-SiFA-based radiopharmaceuticals in PET nuclear imaging. Bioconjugate Chem 27, 267–279 (2016).
Perrin, D. M. [18F]-Organotrifluoroborates as radioprosthetic groups for PET Imaging: from design principles to preclinical applications. Acc. Chem. Res. 49, 1333–1343 (2016).
Pourghiasian, M. et al. 18F-AmBF3-MJ9: a novel radiofluorinated bombesin derivative for prostate cancer imaging. Bioorg. Med. Chem. 23, 1500–1506 (2015).
Bernard-Gauthier, V. et al. Recent advances in 18F radiochemistry: a focus on B-18F, Si-18F, Al-18F, and C-18F radiofluorination via spirocyclic iodonium ylides. J. Nucl. Med. 59, 568–572 (2018).
Laverman, P., McBride, W. J., Sharkey, R. M., Goldenberg, D. M. & Boerman, O. C. Al18F labeling of peptides and proteins. J. Labelled Compd. Radiopharm. 57, 219–223 (2014).
Laverman, P. et al. A novel facile method of labeling octreotide with 18F. J. Nucl. Med. 51, 454–461 (2010).
Pauwels, E. et al. Al18F-NOTA-octreotide: first comparison with 68Ga-DOTATATE in a neuroendocrine tumour patient. Eur. J. Nucl. Med. Mol. Imaging 46, 2398–2399 (2019).
Hong, H. et al. Rapid one-step 18F-radiolabeling of biomolecules in aqueous media by organophosphine fluoride acceptors. Nat. Commun. 10, 989 (2019).
Wessmann, S. H., Henriksen, G. & Wester, H.-J. Cryptate mediated nudeophilic 18F-fluorination without azeotropic drying. Nuklearmedizin 51, 1–8 (2012).
Wängler, C. et al. One-step 18F-labeling of carbohydrate-conjugated octreotate-derivatives containing a silicon-fluoride-acceptor (SiFA): in vitro and in vivo evaluation as tumor imaging agents for positron emission tomography (PET). Bioconjugate Chem 21, 2289–2296 (2010).
Dialer, L. O. et al. Studies toward the development of new silicon-containing building blocks for the direct 18F-labeling of peptides. J. Med. Chem. 56, 7552–7563 (2013).
Höhne, A. et al. Synthesis, 18F-labeling, and in vitro and in vivo studies of bombesin peptides modified with silicon-based building blocks. Bioconjugate Chem 19, 1871–1879 (2008).
Lindner, S. et al. Synthesis and in vitro and in vivo evaluation of SiFA-tagged bombesin and RGD peptides as tumor imaging probes for positron emission tomography. Bioconjugate Chem. 25, 738–749 (2014).
Niedermoser, S. et al. In vivo evaluation of 18F-SiFAlin–modified TATE: a potential challenge for 68Ga-DOTATATE, the clinical gold standard for somatostatin receptor imaging with PET. J. Nucl. Med. 56, 1100–1105 (2015).
Wängler, C. et al. One-step 18F-labeling of peptides for positron emission tomography imaging using the SiFA methodology. Nat. Protoc. 7, 1946–1955 (2012).
Wellings, D. A. & Atherton, E. Standard Fmoc protocols. Methods Enzymol. 289, 44–67 (1997).
Iovkova, L. et al. para‐Functionalized Aryl‐di‐tert‐butylfluorosilanes as potential labeling synthons for 18F radiopharmaceuticals. Chemistry 15, 2140–2147 (2009).
Kostikov, A. P. et al. N-(4-(di-tert-butyl[18F]fluorosilyl)benzyl)-2-hydroxy-N,N-dimethylethylammonium bromide ([18F]SiFAN+Br−): a novel lead compound for the development of hydrophilic SiFA-based prosthetic groups for 18F-labeling. J. Fluorine Chem. 132, 27–34 (2011).
Burke, B. P., Clemente, G. S. & Archibald, S. J. Boron–18F containing positron emission tomography radiotracers: advances and opportunities. Contrast Media Mol. Imaging 10, 96–110 (2015).
Chansaenpak, K., Vabre, B. & Gabbaï, F. P. [18F]-Group 13 fluoride derivatives as radiotracers for positron emission tomography. Chem. Soc. Rev. 45, 954–971 (2016).
Kumar, K. & Ghosh, A. 18F-AlF labeled peptide and protein conjugates as positron emission tomography imaging pharmaceuticals. Bioconjugate Chem 29, 953–975 (2018).
Bernard-Gauthier, V. et al. 18F-labeled silicon-based fluoride acceptors: potential opportunities for novel positron emitting radiopharmaceuticals. Biomed Res. Int. 2014, 454503 (2014).
Acknowledgements
Funding for this research was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC, Discovery Grant) to R.S.
Author information
Authors and Affiliations
Contributions
S.L. and C.W. performed the chemistry/radiochemistry, developed the protocol and helped writing the materials, procedure and troubelshooting paragraphs. C.W., J.J.B., K.J., P.B. and B.W. co-developed the protocol and wrote parts of the protocol. R.S. and B. W. developed the protocol and wrote the abstract and introduction of the protocol.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Bernd Neumaier and Chris Phenix for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Niedermoser, S. et al. J. Nucl. Med. 56, 1100–1105 (2015): http://jnm.snmjournals.org/content/56/7/1100.long
Ilhan, H. et al. Eur. J. Nucl. Med. Mol. Imaging 47, 870–880 (2020): https://link.springer.com/article/10.1007/s00259-019-04501-6
Ilhan, H. et al. Eur. J. Nucl. Med. Mol. Imaging 46, 2400–2401 (2019): https://link.springer.com/article/10.1007%2Fs00259-019-04448-8
Related protocol
Wängler, B. et al. Nat. Prot. 7, 1964–1969 (2012): https://doi.org/10.1038/nprot.2012.111
This protocol is an extension to: Nat. Protoc. 7, 1946–1955 (2012): https://doi.org/10.1038/nprot.2012.109
Supplementary information
Supplementary Information
Supplementary Data.
Rights and permissions
About this article
Cite this article
Lindner, S., Wängler, C., Bailey, J.J. et al. Radiosynthesis of [18F]SiFAlin-TATE for clinical neuroendocrine tumor positron emission tomography. Nat Protoc 15, 3827–3843 (2020). https://doi.org/10.1038/s41596-020-00407-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-00407-y
This article is cited by
-
Good practices for the automated production of 18F-SiFA radiopharmaceuticals
EJNMMI Radiopharmacy and Chemistry (2023)
-
Next-generation PET/CT imaging in meningioma—first clinical experiences using the novel SSTR-targeting peptide [18F]SiTATE
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
Favorable SSTR subtype selectivity of SiTATE: new momentum for clinical [18F]SiTATE PET
EJNMMI Radiopharmacy and Chemistry (2022)
-
Good practices for 68Ga radiopharmaceutical production
EJNMMI Radiopharmacy and Chemistry (2022)
-
Protocols for clinical use at Nature Protocols
Nature Protocols (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.