Abstract
Purpose
To develop a bilayer tablet containing sustained-release (SR) nicorandil and immediate-release (IR) atorvastatin components in separate layers for the effective treatment of angina accompanied by hyperlipidemia with less dosing frequency and improved patient compliance.
Methods
Sustained-release nicorandil layer was formulated using HPMC K100M with or without Eudragit RS PO and Ethyl Cellulose 10FP. An immediate-release atorvastatin layer was developed using crospovidone and sodium carbonate. The formulations were optimized using a central composite design with the help of Design Expert software. The bilayer tablets were manufactured using a double-compression technique. Dissolution test study was conducted using three different media (pH 1.2, 4.5, and pH 6.8 buffers). Dissolution profiles were analyzed through model-dependent and model-independent methods using DDSolver, an Excel-based add-in program. Selected formulations were subjected to accelerated stability study according to ICH guidelines.
Results
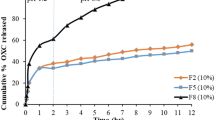
The bilayer tablets were successfully developed with desired quality attributes. Nicorandil release kinetics followed anomalous diffusion, and in vitro release profile was best expressed through the Higuchi equation, while atorvastatin release exhibited Weibull kinetics. The shelf-life of optimized formulations was found to be about 13 months.
Conclusion
The combination of ethyl cellulose and HPMC K100M successfully controlled the release of nicorandil from the bilayer tablet containing immediate-release atorvastatin. The formulation can be subjected to pharmacokinetic studies and clinical trials in the future for better management of cardiovascular diseases and enhancing patient compliance.







Similar content being viewed by others
References
Fu Q, Su X, Hou Y, Li M, Li J, Sun J, et al. Once-daily amoxicillin immediate-and extended-release bilayer tablets. Powder Technol. 2016;301:405–11.
Akseli I, Abebe A, Sprockel O, Cuitiño AM. Mechanistic characterization of bilayer tablet formulations. Powder Technol. 2013;236:30–6.
Perk J, De Backer G, Gohlke H, Graham I, Reiner Ž, Verschuren M, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). Eur Heart J. 2012;33(13):1635–701.
Waeber B, Feihl F, Ruilope LM. Fixed-dose combinations as initial Therapy for Hypertension. Drugs. 2009;69(13):1761–76.
Henderson RA, O'Flynn N. Management of stable angina: summary of NICE guidance. Heart. 2012;98(6):500–7.
Chang L, Li L, Zhang M, Ming-ke N. The myocardial protection of nicorandil plus atorvastatin treatment during percutaneous coronary intervention (PCI). Chinese Journal of Hospital Pharmacy. 2012;21:019.
Frydman AM, Chapelle P, Diekmann H, Bruno R, Thebault JJ, Bouthier J, et al. Pharmacokinetics of nicorandil. Am J Cardiol. 1989;63(21):J25–33.
Camm AJ, Maltz MB. A controlled single-dose study of the efficacy, dose response and duration of action of nicorandil in angina pectoris. Am J Cardiol. 1989;63(21):J61–J5.
Bravo SA, Lamas MC, Salomón CJ. In-vitro studies of diclofenac sodium controlled-release from biopolymeric hydrophilic matrices. J Pharm Pharm Sci. 2002;5(3):213–9.
Mehta KA, Kislalioglu M, Phuapradit W, Malick A, Shah N. Release performance of a poorly soluble drug from a novel, Eudragit®-based multi-unit erosion matrix. Int J Pharm. 2001;213(1):7–12.
Chithaluru K, Tadikonda R, Gollapudi R, Kandula KKK. Formulation and invitro evaluation of sustained release matrix tablets of losartan potassium. Cellulose. 2011;1200:200.
Lakade S, Bhalekar M. Formulation and evaluation of sustained release matrix tablet of anti-anginal drug, influence of combination of hydrophobic and hydrophlic matrix former. Pharm Technol. 2008;1(4):410–3.
Kasu RRR, Mutalik S, Reddy S. Correction to: Once-daily sustained-release matrix tablets of nicorandil: formulation and in vitro evaluation. AAPS PharmSciTech. 2020;21(5):197.
Reza MS, Quadir MA, Haider SS. Comparative evaluation of plastic, hydrophobic and hydrophilic polymers as matrices for controlled-release drug delivery. J Pharm Pharm Sci. 2003;6(2):282–91.
Tamilvanan S, Venkatesh Babu R, Nappinai A, Sivaramakrishnan G. In vitro and in vivo evaluation of hydrophilic and hydrophobic polymers-based nicorandil-loaded peroral tablet compared with its once-daily commercial sustained-release tablet. Drug Dev Ind Pharm. 2011;37(4):436–45.
Patil A, Pohane A, Darbar R, Koutika S, Pothanganti A. Formulation and evaluation of sustained release matrix tablets of Nicorandil. Int J Appl Biol Pharm Technol. 2011;2(3):242–6.
Iffat WSM, Yousuf RI. Hydrophillic and Hydrophobic Polymer Combination: Formulation Development and Optimatization of Nicorandil Sustained Release Tablet. Lat Am J Pharm. 2017;36(5):907–17.
Ahjel SW, Lupuleasa D. Enhancement of solubility and dissolution rate of different forms of atorvastatin calcium in direct compression tablet formulas. Farmacia. 2009;57:290–300.
Mukharya A, Chaudhary S, Patel N, Mansuri N, Kumar A. Stable and bio-equivalent formulation of HMG-CoA reductase inhibitor: Atorvastatin Calcium. Int J Pharm Sci Lett. 2011;2(1):12–20.
Solanki UMDD, Bhimani BB, Patel G, Patel UP, Shah RJ. Formulation and evaluation of bilayer gastro retentive drug delivery system of nicorandil. Int J Health Pharm Sci. 2013;2(1):33–43.
Bashir S, Sherwani M, Shabbir I, Batool A. Efficacy of fix dose combination (atorvastatin and amlodipine) in treatment of uncontrolled hypertension and dyslipidemia. J Ayub Med Coll Abbottabad. 2011;23(3):97–100.
USP 35 NF 30: United States Pharmacopeial Convention Rockville (MD). 2012;868–70.
Rashid A, Ahmad M, Tulain UR, Iqbal FM. Fabrication and evaluation of 2-hydroxyethyl methacrylate-co-acrylic acid hydrogels for sustained nicorandil delivery. Trop J Pharm Res. 2015;14(7):1121–8.
Carstensen J. Stability of solids and solid dosage forms. J Pharm Sci. 1974;63(1):1–14.
Rawlins E. Bentley’s textbook of pharmaceutics. London. England: Cassell and Collier MacMillan; 1977.
Frampton JE, Buckley MM, Fitton A. Nicorandil. Drugs. 1992;44(4):625–55.
Andersson K. Clinical pharmacology of potassium channel openers. Pharmacol Toxicol. 1984;70:411.
Mirani AG, Patravale VB. Design of experiments: basic concepts and its application in pharmaceutical product development. In: Pharmaceutical Product Development. Boca Raton: CRC Press; 2016. p. 130–175.
Ahmed FR, Shoaib MH, Yousuf RI, Ali T, Geckeler KE, Siddiqui F, et al. Clay nanotubes as a novel multifunctional excipient for the development of directly compressible diclofenac potassium tablets in a SeDeM driven QbD environment. Eur J Pharm Sci. 2019;133:214–27.
Capece M, Huang Z, Dave R. Insight into a novel strategy for the design of tablet formulations intended for direct compression. J Pharm Sci. 2017;106(6):1608–17.
Cao Q-R, Choi Y-W, Cui J-H, Lee B-J. Formulation, release characteristics and bioavailability of novel monolithic hydroxypropylmethylcellulose matrix tablets containing acetaminophen. J Control Release. 2005;108(2):351–61.
Rahim N, Naqvi SBS, Bibi R, Iffat W, Shakeel S, Muhammad IN. Disintegrants combination: Development and optimization of a cefadroxil fast disintegrating tablet. Pak J Pharm Sci. 2014;27(5 Spec no):1467–75.
Shirsand S, Suresh S, Swamy P, Para M, Kumar DN. Formulation design of fast disintegrating tablets using disintegrant blends. Indian J Pharm Sci. 2010;72(1):130.
Iffat WSM, Yousuf RI, Rahim N, Sultana A, Maboos M. Application and validation of analytical method for simultaneous determination of nicorandil and atorvastatin in bulk and tablet formulations. Lat Am J Pharm. 2018;37(8):1510–6.
Pharmacopoeia J. Society of Japanese Pharmacopoeia. Amended Chapters. 2007;35(35.2):7.
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, et al. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12(3):263–71.
Siepmann J, Siepmann F. Mathematical modeling of drug delivery. Int J Pharm. 2008;364(2):328–43.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15(1):25–35.
Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur J Pharaceutical Sci. 2001;13(2):123–33.
Shoaib MH, Siddiqi SAS, Yousuf RI, Zaheer K, Hanif M, Rehana S, et al. Development and evaluation of hydrophilic colloid matrix of famotidine tablets. AAPS PharmSciTech. 2010;11(2):708–18.
Moore JW, Flanner HH. Mathematical comparison of dissolution profiles. Pharm Technol. 1996;20(6):64–74.
Anderson N, Bauer M, Boussac N, Khan-Malek R, Munden P, Sardaro M. An evaluation of fit factors and dissolution efficiency for the comparison of in vitro dissolution profiles. J Pharm Biomed Anal. 1998;17(4):811–22.
ICH, Draft Revised Guidance. “Stability testing of new drug substances and products Q1A (R2).” In: Proceedings of the International Conference on Harmonization. Geneva, Switzerland. 2003.
Krishna SRDM. Formulation, optimization and in-vitro charcterization of the sustained release matrix tablets of nicorandil. Int J CurrResChemPharmaSci. 2014;1(10):22–44.
Pharmacopoeia B, Commission BP. 2013.
Jain SP, Mehta DC, Shah SP, Singh PP, Amin PD. Melt-in-mouth pellets of fexofenadine hydrochloride using crospovidone as an extrusion–spheronisation aid. AAPS PharmSciTech. 2010;11(2):917–23.
Perissutti B, Rubessa F, Moneghini M, Voinovich D. Formulation design of carbamazepine fast-release tablets prepared by melt granulation technique. Int J Pharm. 2003;256(1):53–63.
Patil C, Das S. Effect of various superdisintegrants on the drug release profile and disintegration time of Lamotrigine orally disintegrating tablets. Afr J Pharm Pharmacol. 2009;5(1):76–82.
Tiwari SB, Murthy TK, Pai MR, Mehta PR, Chowdary PB. Controlled release formulation of tramadol hydrochloride using hydrophilic and hydrophobic matrix system. AAPS PharmSciTech. 2003;4(3):18–23.
Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010;67(3):217–23.
Prajapati G, Patel R. Design and in vitro evaluation of novel nicorandil sustained release matrix tablets based on combination of hydrophillic and hydrophobic matrix systems. Int J Pharm Sci Rev Res. 2010;1:33–5.
Wadher KJ, Kakde RB, Umekar MJ. Study on sustained-release metformin hydrochloride from matrix tablet: influence of hydrophilic polymers and in vitro evaluation. Int J Pharm Investig. 2011;1(3):157.
Mohapatra S, Kar RK, Sahoo SK. Goodness of fit Model dependent approaches of controlled release matrix tablets of zidovudine. Indian J Pharm Educ Res. 2016;50(1):138–45.
Aksu B, Yurdasiper A, Ege MA, Karasulu HY. Development and comparative evaluation of extended release indomethacin capsules. Afr J Pharm Pharmacol. 2013;7(30):2201–9.
Pahade MA, Jadhav V, Kadam V. Formulation and development of sustained release matrix tablet of nicorandil. Int J Pharm Sci Rev Res. 2010;4(1):107–11.
Popy FA, Dewan I, Parvin MN, Islam SA. Evaluation of in vitro equivalence for tablets containing the poorly water-soluble compound atorvastatin. Dissolution Technologies. 2012;19(4):30–3.
Iffat W, Shoaib MH, Rahim N, Arshad M, Anwer S, Maboos M, et al. Assessment of pharmaceutical equivalence of different generic atorvastatin tablets available in pakistan. Lat Am J Pharm. 2017;36(10):2098–104.
Hooper D, Clarke F, Mitchell J, Snowden M. A modern approach to the Heckel Equation: The effect of compaction pressure on the yield pressure of ibuprofen and its sodium salt. J Nanomed Nanotechnol. 2016;7(381):2.
Gaikwad SS, Chafle SA, Morris PS, Avari JG. Development and evaluation of bilayer tablets of combination of antibiotics for the treatment of sexually transmitted disease. Brazilian J Pharm Sci. 2016;52(3):555–66.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Figure S1:
FT-IR spectrum of (a) Nicorandil Standard (b) HPMC K100M- EUDRAGIT RS PO® Combination (c) HPMC K100M-EC combination (d) Atorvastatin Standard (e) Atorvastatin optimized formulation (f) Bilayer Tablet containing HPMC K100M-EUDRAGIT RS PO® Combination (g) Bilayer tablet containing HPMC K100M-EC10FP Combination (PNG 375 kb)
Rights and permissions
About this article
Cite this article
Iffat, W., Shoaib, M.H., Yousuf, R.I. et al. Use of Eudragit RS PO, HPMC K100M, Ethyl Cellulose, and Their Combination for Controlling Nicorandil Release from the Bilayer Tablets with Atorvastatin as an Immediate-Release Layer. J Pharm Innov 17, 429–448 (2022). https://doi.org/10.1007/s12247-020-09513-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-020-09513-6