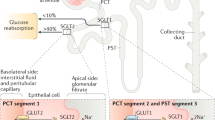
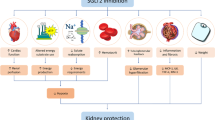
Abstract
Purpose of Review
The micro/macrovascular complications of diabetes cause considerable morbidity and premature mortality. The SGLT2 inhibitors are the first diabetes medications with significant benefits on microvascular disease (nephropathy) and macrovascular cardiovascular disease. In this review, we evaluate one of the potential mechanisms for these cardiorenal benefits—the production of ketones, their benefits, and risks.
Recent Findings
In recent cardiovascular outcome trials (CVOTs), the SGLT2 inhibitors demonstrated significant cardiorenal benefits and they are now approved to reduce CV events/death, heart failure hospitalization, and progression to end-stage renal disease. Glucosuria induced by the SGLT2 inhibitors leads to increased ketone production. Ketones are an efficient fuel source and can improve myocardial and renal function. Further, the ketone body beta-hydroxybutyrate exhibits anti-inflammatory/anti-oxidative actions, which favorably impact myocardial and renal remodeling/fibrosis. Uncontrolled ketogenesis leads to ketoacidosis, especially during conditions of acute illness and excessive insulin dose reductions.
Summary
The SGLT2 inhibitors have demonstrated significant cardiorenal benefits in large CVOTs. Studies are in progress to elucidate whether SGLT2 inhibitor–induced low-grade hyperketonemia contributes to these benefits.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Lau DC. Are diabetes care providers too glucocentric? Can J Diabetes. 2016;40(6):479–81.
The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
•• McMurray JJV, DAPA-HF Trial Committees and Investigators, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008 First study to demonstrate that an SGLT2 inhibitor can reduce HF hospitalization/CV mortality in patients with HFrEF irrespective of diabetes status.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306.
Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS program randomized clinical trials. Lancet Diabetes Endocrinol. 2018;6:691–704.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34.
Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomized trial. Lancet Diabetes Endocrinol. 2019;7:606–17.
Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39:1115–22.
Ferrannini E, et al. Shift to fatty substrate utilization in response to sodium–glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–5.
•• Cahill GF. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22 Comprehensive review of fuel metabolism in starvation.
Cahill GF Jr, Veech RL. Ketoacids? Good medicine? Trans Am Clin Climatol Assoc. 2003;114:149–63.
Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999;15(6):412–26.
Felig P, Lynch V. Starvation in human pregnancy: hypoglycemia, hypoinsulinemia, and hyperketonemia. Science. 1970;170(3961):990–2.
Ferrannini E. Sodium-glucose cotransporters and their inhibition: clinical physiology. Cell Metab. 2017;26:27–38.
Polidori D, Iijima H, Goda M, Maruyama N, Inagaki N, Crawford PA. Intra- and inter-subject variability for increases in serum ketone bodies in patients with type 2 diabetes treated with the sodium glucose co-transporter 2 inhibitor canagliflozin. Diabetes Obes Metab. 2018;20(5):1321–6.
Yabe D, Iwasaki M, Kuwata H, Haraguchi T, Hamamoto Y, Kurose T, et al. Sodium-glucose co-transporter-2 inhibitor use and dietary carbohydrate intake in Japanese individuals with type 2 diabetes: a randomized, open-label, 3-arm parallel comparative, exploratory study. Diabetes Obes Metab. 2017;19:739–43.
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. Sabatine MS.SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–9.
Hupfeld C, Mudaliar S. Navigating the “MACE” in cardiovascular outcomes trials and decoding the relevance of atherosclerotic cardiovascular disease benefits versus heart failure benefits. Diabetes Obes Metab. 2019;21(8):1780–9.
FDA approves new treatment for a type of heart failure. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-type-heart-failure. Accessed June 27, 2020.
Qiangwei F, Colgan SP, Shelley CS. Hypoxia: the force that drives chronic kidney disease. Clin Med Res. 2016;14(1):15–39.
Abe H, Semba H, Takeda N. The roles of hypoxia signaling in the pathogenesis of cardiovascular diseases. J Atheroscler Thromb. 2017;24(9):884–94.
Cho YI, Mooney MP, Cho DJ. Hemorheological disorders in diabetes mellitus. J Diabetes Sci Technol. 2008;2:1130–8.
Pu LJ, Shen Y, Lu L, Zhang RY, Zhang Q, Shen WF. Increased blood glycohemoglobin A1c levels lead to overestimation of arterial oxygen saturation by pulse oximetry in patients with type 2 diabetes. Cardiovasc Diabetol. 2012;11:110.
De Jong KA, Lopaschuk GD. Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Can J Cardiol. 2017;33(7):860–71.
Karwi QG, Uddin GM, Ho KL, Lopaschuk GD. Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med. 2018;5:68.
Okar DA. Starvation amidst plenty: PDKs and diabetes mellitus. Trends Endocrinol Metab. 2002;13:142.
Abel ED, O'Shea KM, Ramasamy R. Insulin resistance: metabolic mechanisms and consequences in the heart. Arterioscler Thromb Vasc Biol. 2012;32(9):2068–76.
Levelt E, Rodgers CT, Clarke WT, Mahmod M, Ariga R, Francis JM, et al. Cardiac energetics, oxygenation, and perfusion during increased workload in patients with type 2 diabetes mellitus. Eur Heart J. 2016;37(46):3461–9.
Pascual F, Coleman RA. Fuel availability and fate in cardiac metabolism: a tale of two substrates. Biochim Biophys Acta. 2016;1861(10):1425–33.
Lahnwong S, Palee S, Apaijai N, Sriwichaiin S, Kerdphoo S, Jaiwongkam T, et al. Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury. Cardiovasc Diabetol. 2020;19(1):91.
Moellmann J, Klinkhammer BM, Droste P, et al. Empagliflozin improves left ventricular diastolic function of db/db mice [published online ahead of print, 2020 Apr 28]. Biochim Biophys Acta Mol basis Dis. 1866;2020(8):165807.
Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Ishikawa K, Watanabe S, Picatoste B, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73(15):1931–44.
•• Nielsen R, Møller N, Gormsen LC, et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation. 2019;139(18):2129–41 First study in humans to show that BHOB infusion may potentially constitute a novel treatment principle in HFrEF patients.
Oldgren J, Laurila S, Akerblom A, et al. Effects of 6 weeks of treatment with dapagliflozin, a sodium-glucose cotransporter 2 inhibitor, on myocardial function and metabolism in type 2 diabetes patients: a randomized placebo-controlled study. J Am Coll Cardiol. 2020;75(11):1610.
EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Preserved Ejection Fraction (EMPEROR-Preserved). https://clinicaltrials.gov/ct2/show/NCT03057951. Accessed 27 June 2020.
Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure. (DELIVER). https://clinicaltrials.gov/ct2/show/NCT03619213. Accessed 27 June 2020.
Hansell P, Welch WJ, Blantz RC, Palm F. Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin Exp Pharmacol Physiol. 2013;40(2):123–37.
Fine LG, Orphanides C, Norman JT. Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int Suppl. 1998;65:S74–8.
Friederich-Persson, et al. Kidney hypoxia, attributable to increased oxygen consumption, induces nephropathy independently of hyperglycemia and oxidative stress. Hypertension. 2013;62:914–9.
Körner A, Eklöf AC, Celsi G, Aperia A. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes. 1994;43:629–33.
Muller ME, Pruijm M, Bonny O, Burnier M, Zanchi A. Effects of the SGLT-2 inhibitor empagliflozin on renal tissue oxygenation in non-diabetic subjects: a randomized, double-blind, placebo-controlled study protocol. Adv Ther. 2018;35(6):875–85.
Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017;25:262–84.
Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease and therapeutics. Nat Med. 2015;21:677–87.
McKellar GE, et al. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat Rev Cardiol. 2009;6:410–7.
Chakraborty S, Galla S, Cheng X, Yeo JY, Mell B, Singh V, et al. Salt-responsive metabolite, beta-hydroxybuterate, attenuates hypertension. Cell Rep. 2018;25(3):677–89.
Kim SR, Lee SG, Kim SH, Kim JH, Choi E, Cho W, et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020;11:2127.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–70.
Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol. 2014;6(4):a018713.
Ooi JY, Tuano NK, Rafehi H, et al. HDAC inhibition attenuates cardiac hypertrophy by acetylation and deacetylation of target genes. Epigenetics. 2015;10(5):418–30.
Verma, EMPA-HEART CardioLink-6 Investigators, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease. Circulation. 2019;140:1693–702.
Bami K, Gandhi S, Leong-Poi H, Yan AT, Ho E, Zahrani M, et al. Effects of empagliflozin on left ventricular remodeling in patients with type 2 diabetes and coronary artery disease: echocardiographic substudy of the EMPA-HEART CardioLink-6 randomized clinical trial. J Am Soc Echocardiogr. 2020;33(5):644–6.
Wang X, Liu J, Zhen J, Zhang C, Wan Q, Liu G, et al. Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney Int. 2014;86:712–25.
Hadden MJ, Advani A. Histone deacetylase inhibitors and diabetic kidney disease. Int J Mol Sci. 2018;19:2630.
Umino H, Hasegawa K, Minakuchi H, Muraoka H, Kawaguchi T, Kanda T, et al. High basolateral glucose increases sodium-glucose cotransporter 2 and reduces sirtuin-1 in renal tubules through glucose transporter-2 detection. Sci Rep. 2018;8(1):6791.
Fayfman M, Pasquel FJ, Umpierrez GE. Management of hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med Clin North Am. 2017;101(3):587–606.
Bonora BM, Avogaro A, Fadini GP. Euglycemic ketoacidosis. Curr Diab Rep. 2020;20:25.
Rosenstock J, Ferranini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38:1638–42.
Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thévenet J, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015;21(5):512–7.
Hodson DJ, Rorsman P. A variation on the theme: SGLT2 inhibition and glucagon secretion in human islets. Diabetes. 2020;69(5):864–6.
First oral add-on treatment to insulin for treatment of certain patients with type 1 diabetes. https://www.ema.europa.eu/en/news/first-oral-add-treatment-insulin-treatment-certain-patients-type-1-diabetes. Accessed June 27,2020.
Liu J, Li L, Li S, et al. Sodium-glucose co-transporter-2 inhibitors and the risk of diabetic ketoacidosis in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials [published online ahead of print, 2020 May 4]. Diabetes Obes Metab. 2020. https://doi.org/10.1111/dom.14075.
Wang L, Voss EA, Weaver J, Hester L, Yuan Z, DeFalco F, et al. Diabetic ketoacidosis in patients with type 2 diabetes treated with SGLT2- inhibitors versus other antihyperglycemic agents: an observational study of four US administrative claims databases. Pharmacoepidemiol Drug Saf. 2019;28(12):1620–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pharmacologic Treatment of Type 2 Diabetes
Rights and permissions
About this article
Cite this article
Ekanayake, P., Hupfeld, C. & Mudaliar, S. Sodium-Glucose Cotransporter Type 2 (SGLT-2) Inhibitors and Ketogenesis: the Good and the Bad. Curr Diab Rep 20, 74 (2020). https://doi.org/10.1007/s11892-020-01359-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s11892-020-01359-z