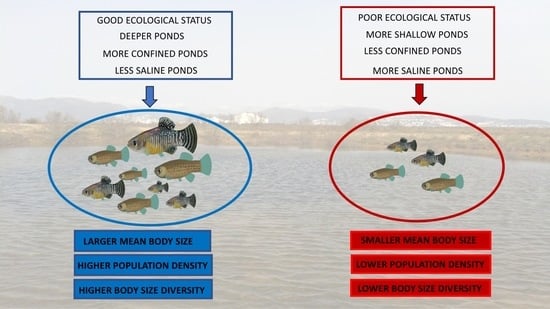
Factors Influencing Abundances and Population Size Structure of the Threatened and Endemic Cyprinodont Aphanius iberus in Mediterranean Brackish Ponds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Sampling and Analysis
2.3. Aphanius iberus Abundance and Size Structure
2.4. Data Analysis
3. Results
3.1. Description of the Local Characteristics in the Mediterranean Ponds
3.2. Variation of the Population Structure of A. iberus across Mediterranean Ponds
3.3. Main Drivers Affecting the Fish Population Size Structure and Density
3.4. Influence of the Presence of G. holbrooki on the Size Structure and Density of A. iberus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doadrio, I. Atlas y Libro Rojo de los Peces Continentals de España; Ministerio del medio ambiente: Madrid, Spain, 2001. [Google Scholar]
- Vilà, M.; García-Berthou, E. Monitoring biological invasions in freshwater habitats. In Conservation Monitoring in Freshwater Habitats a Practical Guide and Case Studies; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 9781402092770. [Google Scholar]
- Moreno-Amich, R. Els peixos d’aigua dolça i salabrosa. In Els Aiguamolls de l’Empordà Aspectes Ecologics, Històrics i Socials del Parc Natural; Caries, V., Sargatal, J., Felix, J., Eds.; Editorial ART-3: Figueres, Spain, 1989; pp. 241–250. [Google Scholar]
- De Sostoa, A. Las Comunidades de Peces del Delta del Ebro. Ph.D. Thesis, University of Barcelona, Barcelona, Spain, 1983. [Google Scholar]
- Oliva-Paterna, F.J.; Torralva, M.; Fernández-Delgado, C. Threatened fishes of the world: Aphanius iberus (Cuvier & Valenciennes, 1846) (Cyprinodontidae). Environ. Biol. Fishes 2006. [Google Scholar] [CrossRef]
- Doadrio, I.; Carmona, J.A.; Fernández-Delgado, C. Morphometric study of the Iberian Aphanius (Actinopterygii, Cyprinodontiformes), with description of a new species. Folia Zool. 2002, 51, 67–79. [Google Scholar]
- Crivelli, A.J. Aphanius Iberus; The IUCN Red List of Threatened Species. 2006. Available online: http://www.iucnredlist.org (accessed on 25 May 2018).
- García-Berthou, E.; Moreno-Amich, R. Age and growth of an Iberian cyprinodont, Aphanius iberus (Cuv. & Val.), in its most northerly population. J. Fish. Biol. 1992. [Google Scholar] [CrossRef]
- García-Berthou, E.; Moreno-Amich, R. Multivariate analysis of covariance in morphometric studies of the reproductive cycle. Can. J. Fish. Aquat. Sci. 1993. [Google Scholar] [CrossRef]
- García-Berthou, E.; Moreno-Amich, R. Ecología y conservación del fartet (Lebias ibera) en las marismas del Ampurdán (Cataluña). In Peces Ciprinodóntidos Ibéricos. Fartet y Samaruc; Generalitat Valenciana, Conselleria del medio Ambiente: Valencia, Spain, 1999; pp. 151–161. [Google Scholar]
- Demestre, M.; Roig, A.; De Sostoa, A.; De Sostoa, F. Contribució a l’estudi de la ictiofauna continental del delta de I’Ebre. Treb. Inst. Cat. Hist. Nat. 1977, 8, 145–226. [Google Scholar]
- Rincón, P.A.; Correas, A.M.; Morcillo, F.; Risueño, P.; Lobón-Cerviá, J. Interaction between the introduced eastern mosquitofish and two autochthonous Spanish toothcarps. J. Fish. Biol. 2002. [Google Scholar] [CrossRef]
- Mack, R.N.; Simberloff, D.; Lonsdale, W.M.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Casas, J.J.; Sánchez-Oliver, J.S.; Sanz, A.; Furné, M.; Trenzado, C.; Juan, M.; Paracuellos, M.; Suárez, M.D.; Fuentes, F.; Gallego, I.; et al. The paradox of the conservation of an endangered fish species in a Mediterranean region under agricultural intensification. Biol. Conserv. 2011. [Google Scholar] [CrossRef]
- Franco, A.; Torricelli, P.; Franzoi, P. A habitat-specific fish-based approach to assess the ecological status of Mediterranean coastal lagoons. Mar. Pollut. Bull. 2009. [Google Scholar] [CrossRef]
- Prado, P.; Alcaraz, C.; Jornet, L.; Caiola, N.; Ibańẽz, C. Effects of enhanced hydrological connectivity on mediterranean salt marsh fish assemblages with emphasis on the endangered Spanish toothcarp(Aphanius iberus). Peer J. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmgren, K.; Appelberg, M. Size structure of benthic freshwater fish communities in relation to environmental gradients. J. Fish. Biol. 2000. [Google Scholar] [CrossRef]
- Emmrich, M.; Brucet, S.; Ritterbusch, D.; Mehner, T. Size spectra of lake fish assemblages: Responses along gradients of general environmental factors and intensity of lake-use. Freshw. Biol. 2011, 56, 2316–2333. [Google Scholar] [CrossRef]
- Arranz, I.; Mehner, T.; Benejam, L.; Argillier, C.; Holmgren, K.; Jeppesen, E.; Lauridsen, T.L.; Volta, P.; Winfield, I.J.; Brucet, S.; et al. Density-dependent effects as key drivers of intraspecific size structure of six abundant fish species in lakes across Europe 1. Can. J. Fish. Aquat. Sci. 2016, 73, 519–534. [Google Scholar] [CrossRef] [Green Version]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World Worst Invasive Alien Species. A Selection from the Global Invasive Species Database; The Invasive Species Specialist Group: Auckland, New Zeland, 2020. [Google Scholar]
- Pyke, G.H. A review of the biology of Gambusia affinis and G. holbrooki. Rev. Fish. Biol. Fish. 2005, 15, 339–365. [Google Scholar] [CrossRef]
- Vargas, M.J. Interaccion entre Aphanius Iberus y Gambusia Holbrooki en el Helta del Ebro: Sus Ciclos Biologicos y Ecologıas Troficas. Ph.D. Thesis, Universitat de Barcelona, Barcelona, Spain, 1993. [Google Scholar]
- Alcaraz, C.; García-Berthou, E. Life history variation of invasive mosquitofish (Gambusia holbrooki) along a salinity gradient. Biol. Conserv. 2007. [Google Scholar] [CrossRef]
- Haiahem, D.; Touati, L.; Baaziz, N.; Samraoui, F.; Alfarhan, A.H.; Samraoui, B. Impact of eastern mosquitofish, Gambusia holbrooki, on temporary ponds: Insights on how predation may structure zooplankton communities. Zool. Ecol. 2017. [Google Scholar] [CrossRef]
- Crivelli, A.; Boy, V. The diet of the mosquitofish Gambusia affinis (Baird & Girard) (Poecilliidae) in Mediterranean France. Rev. d’Ecologie 1987, 42, 421–435. [Google Scholar]
- Garcia-Berthou, E. Food of introduced mosquitofish: Ontogenetic diet shift and prey selection. J. Fish. Biol. 1999. [Google Scholar] [CrossRef]
- Alcaraz, C.; Bisazza, A.; García-Berthou, E. Salinity mediates the competitive interactions between invasive mosquitofish and an endangered fish. Oecologia 2008, 155, 205–213. [Google Scholar] [CrossRef]
- Caiola, N.; De Sostoa, A. Possible reasons for the decline of two native toothcarps in the Iberian Peninsula: Evidence of competition with the introduced Eastern mosquitofish. J. Appl. Ichthyol. 2005, 21, 358–363. [Google Scholar] [CrossRef]
- Alcaraz, C.; Pou-Rovira, Q.; García-Berthou, E. Use of a flooded salt marsh habitat by an endangered cyprinodontid fish (Aphanius iberus). Hydrobiologia 2008. [Google Scholar] [CrossRef]
- Magellan, K.; García-Berthou, E. Experimental evidence for the use of artificial refugia to mitigate the impacts of invasive Gambusia holbrooki on an endangered fish. Biol. Invasions 2016. [Google Scholar] [CrossRef]
- Prado, P.; Vergara, C.; Caiola, N.; Ibáñez, C. Influence of salinity regime on the food-web structure and feeding ecology of fish species from Mediterranean coastal lagoons. Estuar. Coast. Shelf Sci. 2014. [Google Scholar] [CrossRef]
- Brett, J.R.; Groves, T.D.D. Physiological energetics. Fish Physiol. 1979, 8, 279–352. [Google Scholar]
- Wootton, R.J. Ecology of Teleost Fishes; Springer: Berlin/Heidelberg, Germany, 1989. [Google Scholar]
- Yildirim, Ö.; Karacuha, A. Effect of salinity on growth performance and survival rate of Aphanius chantrei (Galliard, 1895). J. Appl. Ichthyol. 2008. [Google Scholar] [CrossRef]
- Plaut, I. Resting metabolic rate, critical swimming speed, and routine activity of the euryhaline cyprinodontid, Aphanius dispar, acclimated to a wide range of salinities. Physiol. Biochem. Zool. 2000. [Google Scholar] [CrossRef]
- Oltra, R.; Todolí, R. Reproduction of the endangered killifish Aphanius iberus at different salinities. Environ. Biol. Fishes 2000. [Google Scholar] [CrossRef]
- Vargas, M.J.; De Sostoa, A. Life-history pattern of the Iberian toothcarp Aphanius iberus (Pisces, Cyprinodontidae) from a Mediterranean estuary, the Ebro Delta (Spain). Netherlands J. Zool. 1997. [Google Scholar] [CrossRef] [Green Version]
- Oliva-Paterna, F.J.; García-Alonso, J.; Cardozo, V.; Torralva, M. Field studies of ammonia excretion in Aphanius iberus (Pisces; Cyprinodontidae): Body size and habitat effects. J. Appl. Ichthyol. 2007. [Google Scholar] [CrossRef]
- Quintana, X.D.; Boix, D.; Badosa, A.; Brucet, S.; Compte, J.; Gascon, S.; López-Flores, R.; Sala, J.; Moreno-Amich, R. Community structure in mediterranean shallow lentic ecosystems: Size-based vs. taxon-based approaches. Limnetica 2006, 25, 303–320. [Google Scholar]
- Badosa, A.; Boix, D.; Brucet, S.; López-Flores, R.; Gascón, S.; Quintana, X.D. Zooplankton taxonomic and size diversity in Mediterranean coastal lagoons (NE Iberian Peninsula): Influence of hydrology, nutrient composition, food resource availability and predation. Estuar. Coast. Shelf Sci. 2007, 71, 335–346. [Google Scholar] [CrossRef]
- Compte, J.; Gascón, S.; Quintana, X.D.; Boix, D. The effects of small fish presence on a species-poor community dominated by omnivores: Example of a size-based trophic cascade. J. Exp. Mar. Bio. Ecol. 2012, 418–419, 1–11. [Google Scholar] [CrossRef]
- Quintana, X.D.; Brucet, S.; Boix, D.; López-Flores, R.; Gascón, S.; Badosa, A.; Sala, J.; Moreno-Amich, R.; Egozcue, J.J. A nonparametric method for the measurement of size diversity with emphasis on data standardization. Limnol. Oceanogr. Methods 2008, 6, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Quintana, X.D.; Moreno-Amich, R.; Comín, F.A. Nutrient and plankton dynamics in a Mediterranean salt marsh dominated by incidents of flooding. Part 1: Differential confinement of nutrients. J. Plankton Res. 1998. [Google Scholar] [CrossRef]
- Quintana, X.D.; Comín, F.A.; Moreno-Amich, R. Biomass-size spectra in aquatic communities in shallow fluctuating Mediterranean salt marshes (Empordà wetlands, NE Spain). J. Plankton Res. 2002. [Google Scholar] [CrossRef] [Green Version]
- Sgarzi, S.; Badosa, A.; Leiva-Presa, À.; Benejam, L.; López-Flores, R.; Brucet, S. Plankton taxonomic and size diversity of Mediterranean brackish ponds in spring: Influence of abiotic and biotic factors. Water 2019, 11, 106. [Google Scholar] [CrossRef] [Green Version]
- Badosa, A.; Boix, D.; Brucet, S.; López-Flores, R.; Quintana, X.D. Nutrients and zooplankton composition and dynamics in relation to the hydrological pattern in a confined Mediterranean salt marsh (NE Iberian Peninsula). Estuar. Coast. Shelf Sci. 2006, 66, 513–522. [Google Scholar] [CrossRef]
- Quintana, X.D.; Baix, D.; Badosa, A.; Brucet, S.; Gascón, S.; Gesti, J.; Trobajo, R. Limnología de los humedales costeros mediterráneos. El caso de Aiguamolls de l ’Empordà. Ecosistemas 2004, 13, 30–36. [Google Scholar]
- López-Flores, R.; Boix, D.; Badosa, A.; Brucet, S.; Quintana, X.D. Environmental factors affecting bacterioplankton and phytoplankton dynamics in confined Mediterranean salt marshes (NE Spain). J. Exp. Mar. Bio. Ecol. 2009, 369, 118–126. [Google Scholar] [CrossRef]
- Google. (n.d.). Google Maps Area Calculator Tool. 2014. Available online: https://www.daftlogic.com/projects-google-maps-area-calculator-tool.htm (accessed on 8 January 2019).
- Sen Gupta, R.; Koroleff, F. A quantitative study of nutrient fractions and a stoichiometric model of the Baltic. Estuar. Coast. Mar. Sci. 1973, 1, 335–360. [Google Scholar] [CrossRef]
- Sala, J.; Gascón, S.; Boix, D.; Gesti, J.; Quintana, X.D. Proposal of a rapid methodology to assess the conservation status of Mediterranean wetlands and its application in Catalunya (NE Iberian Peninsula). Arch. Sci. 2004, 57, 141–152. [Google Scholar]
- Boix, D.; Gascón, S.; Sala, J.; Martinoy, M.; Gifre, J.; Quintana, X.D. A new index of water quality assessment in Mediterranean wetlands based on crustacean and insect assemblages: The case of Catalunya (NE Iberian Peninsula). Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, 635–651. [Google Scholar] [CrossRef]
- Blanco, S.; Romo, S.; Villena, M.J.; Martínez, S. Fish communities and food web interactions in some shallow Mediterranean lakes. Hydrobiologia 2003, 506, 473–480. [Google Scholar] [CrossRef]
- Franco, A.; Pérez-Ruzafa, A.; Drouineau, H.; Franzoi, P.; Koutrakis, E.T.; Lepage, M.; Verdiell-Cubedo, D.; Bouchoucha, M.; López-Capel, A.; Riccato, F.; et al. Assessment of fish assemblages in coastal lagoon habitats: Effect of sampling method. Estuar. Coast. Shelf Sci. 2012. [Google Scholar] [CrossRef]
- Pielou, E.C. An Introduction to Mathematical Ecology; Wiley-Interscience: New York, NY, USA, 1969. [Google Scholar]
- Brucet, S.; Tavşanoğlu, Ü.N.; Özen, A.; Levi, E.E.; Bezirci, G.; Çakıroğlu, A.İ.; Jeppesen, E.; Svenning, J.C.; Ersoy, Z.; Beklioğlu, M. Size-based interactions across trophic levels in food webs of shallow Mediterranean lakes. Freshw. Biol. 2017, 62, 1819–1830. [Google Scholar] [CrossRef]
- Brucet, S.; Arranz, I.; Mehner, T.; Argillier, C.; Beklioğlu, M.; Benejam, L.; Boll, T.; Holmgren, K.; Lauridsen, T.L.; Svenning, J.C.; et al. Size diversity and species diversity relationships in fish assemblages of Western Palearctic lakes. Ecography 2018. [Google Scholar] [CrossRef]
- Fletcher, T.D. QuantPsyc: Quantitative Psychology Tools; R Package Version 1.5. 2012. Available online: http://cran.r-project.org/package=QuantPsyc (accessed on 15 July 2019).
- Pinheiro, J.C.; Bates, D.M. Extending the Basic Linear. In Mixed Effect Models in S & S-Plus; Pinheiro, J.C., Bates, D.M., Eds.; Springer Verlag: Rensselaer, NY, USA, 2000; pp. 201–270. [Google Scholar]
- Wickham, H. Ggplot2 Elegant Graphics for Data Analysis (Use R!); R Foundation for Statistical Computing: Vienna, Austria, 2016; ISBN 9780387981406. [Google Scholar]
- Brucet, S.; Boix, D.; López-Flores, R.; Badosa, A.; Moreno-Amich, R.; Quintana, X.D. Zooplankton structure and dynamics in permanent and temporary Mediterranean salt marshes: Taxon-based and size-based approaches. Arch. Hydrobiol. 2005, 162, 535–555. [Google Scholar] [CrossRef]
- Leonardos, I.; Sinis, A. Reproductive strategy of Aphanius fasciatus Nardo, 1827 (Pisces: Cyprinodontidae) in the Mesolongi and Etolikon lagoons (W. Greece). Fish. Res. 1998. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jeppesen, E.; Jensen, J.P. Pond or lake: Does it make any difference? Arch. Hydrobiol. 2005. [Google Scholar] [CrossRef]
- Peters, R.H.; Downing, J.A. Empirical analysis of zooplankton filtering and feeding rates. Limnol. Oceanogr. 1984. [Google Scholar] [CrossRef] [Green Version]
- Brucet, S.; Boix, D.; López-Flores, R.; Badosa, A.; Quintana, X.D. Size and species diversity of zooplankton communities in fluctuating Mediterranean salt marshes. Estuar. Coast. Shelf Sci. 2006, 67, 424–432. [Google Scholar] [CrossRef]
- Quintana, X.D.; Cañedo-Argüelles, M.; Nebra, A.; Gascón, S.; Rieradevall, M.; Caiola, N.; Sala, J.; Ibàñez, C.; Sánchez-Millaruelo, N.; Boix, D. New Tools to Analyse the Ecological Status of Mediterranean Wetlands and Shallow Lakes. In Experiences from Surface Water Quality Monitoring; Springer: Cham, Switzerland, 2015; pp. 171–199. [Google Scholar]


| Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|
| Mean water column depth (cm) | 59.30 | 41.93 | 16.00 | 150.00 |
| Pond area (m2) | 10,873.60 | 20,463.50 | 147.90 | 68,150.00 |
| Conductivity (mS·cm−1) | 46.14 | 17.63 | 10.07 | 69.10 |
| Total nitrogen (μmol·L−1) | 92.01 | 51.63 | 58.55 | 234.40 |
| Zooplakton biomass (µg·L−1) | 498.17 | 1313.92 | 1.13 | 4840.48 |
| ECELS index | 77.00 | 18.70 | 43.00 | 98.00 |
| QAELSe 2010 index | 0.47 | 0.12 | 0.25 | 0.56 |
| Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|
| Aphanius iberus maximum length (mm) | 41.08 | 8.06 | 19.00 | 54.00 |
| Aphanius iberus mean length (mm) | 29.21 | 5.36 | 16.00 | 41.00 |
| Aphanius iberus length range (mm) | 21.67 | 7.38 | 6.00 | 41.00 |
| Aphanius iberus size diversity | 1.15 | 0.36 | 0.27 | 2.34 |
| Aphanius iberus capture per effort unit (CPUE) | 59.49 | 91.68 | 2.00 | 525.00 |
| Response Variable | Predictor | Estimate | S.E. | Beta Coefficients | t-Value | p-Value | df |
|---|---|---|---|---|---|---|---|
| Aphanius iberus MAXIMUM length | Conductivity | −19.49 | 4.69 | −0.47 | −4.15 | <0.01 | 5 |
| Log Total Nitrogen | 2325.09 | 564.20 | 0.45 | 4.12 | <0.01 | 5 | |
| QAELS index | 3894.26 | 690.27 | 0.59 | 5.64 | <0.01 | 5 | |
| Zooplankton biomass | −0.22 | 0.05 | −0.69 | −4.51 | <0.01 | 5 | |
| Aphanius iberus MEAN length | Conductivity | −0.15 | 0.05 | −0.42 | −3.35 | 0.02 | 6 |
| QAELS index | 31.94 | 6.76 | 0.55 | 4.73 | <0.01 | 6 | |
| Zooplankton biomass | −0.01 | −0.01 | −0.59 | −4.01 | 0.01 | 6 | |
| Aphanius iberus length RANGE | Log Pond Mean Depth | 14.45 | 5.36 | 0.42 | 2.70 | 0.03 | 7 |
| Log Total Nitrogen | 36.88 | 8.93 | 0.48 | 4.13 | <0.01 | 7 | |
| Aphanius iberus SIZE DIVERSITY | Conductivity | <0.01 | <0.01 | 0.29 | 2.21 | 0.06 | 7 |
| Log Total Nitrogen | 0.93 | 0.37 | 0.30 | 2.51 | 0.04 | 7 | |
| Aphanius iberus capture per effort unit (CPUE) | Log Pond Mean Depth | 1.46 | 0.57 | 0.55 | 2.56 | 0.04 | 7 |
| Log Total Nitrogen | 2.15 | 0.97 | 0.36 | 2.23 | 0.06 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgarzi, S.; Brucet, S.; Bartrons, M.; Arranz, I.; Benejam, L.; Badosa, A. Factors Influencing Abundances and Population Size Structure of the Threatened and Endemic Cyprinodont Aphanius iberus in Mediterranean Brackish Ponds. Water 2020, 12, 3264. https://doi.org/10.3390/w12113264
Sgarzi S, Brucet S, Bartrons M, Arranz I, Benejam L, Badosa A. Factors Influencing Abundances and Population Size Structure of the Threatened and Endemic Cyprinodont Aphanius iberus in Mediterranean Brackish Ponds. Water. 2020; 12(11):3264. https://doi.org/10.3390/w12113264
Chicago/Turabian StyleSgarzi, Serena, Sandra Brucet, Mireia Bartrons, Ignasi Arranz, Lluís Benejam, and Anna Badosa. 2020. "Factors Influencing Abundances and Population Size Structure of the Threatened and Endemic Cyprinodont Aphanius iberus in Mediterranean Brackish Ponds" Water 12, no. 11: 3264. https://doi.org/10.3390/w12113264