Relationship between Very Fine Root Distribution and Soil Water Content in Pre- and Post-Harvest Areas of Two Coniferous Tree Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
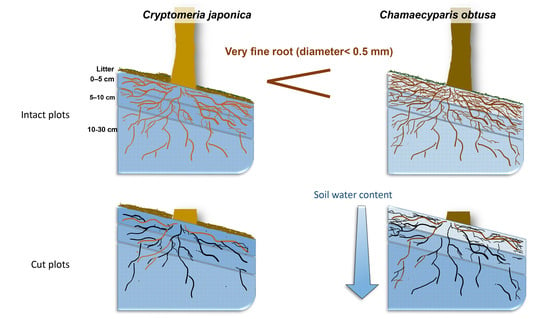
2.2. Sampling Design and Soil Collection
2.3. Experiments
2.3.1. Soil Experiments
2.3.2. Root Experiments
2.4. Statistical Analysis
3. Results
3.1. Soil Volumetric Water Content
3.2. Root Characteristics
3.2.1. Biomass Density
3.2.2. Length Density
3.2.3. Specific Root Length
3.3. Soil Water Content Relationships with Root Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reubens, B.; Poesen, J.; Danjon, F.; Geudens, G.; Muys, B. The role of fine and coarse roots in shallow slope stability and soil erosion control with a focus on root system architecture: A review. Trees 2007, 21, 385–402. [Google Scholar] [CrossRef]
- Makita, N.; Hirano, Y.; Dannoura, M.; Kominami, Y.; Mizoguchi, T.; Ishii, H.; Kanazawa, Y. Fine root morphological traits determine variation in root respiration of Quercus serrata. Tree Physiol. 2009, 29, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Abdi, E.; Deljouei, A. Seasonal and spatial variability of root reinforcement in three pioneer species of the Hyrcanian forest. Austrian J. For. Sci. 2019, 136, 175–198. [Google Scholar]
- Montagnoli, A.; Terzaghi, M.; Di Iorio, A.; Scippa, G.S.; Chiatante, D. Fine-root morphological and growth traits in a Turkey-oak stand in relation to seasonal changes in soil moisture in the Southern Apennines, Italy. Ecol. Res. 2012, 27, 1015–1025. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Mao, Z.; Sun, T. Condensed tannin effects on decomposition of very fine roots among temperate tree species. Soil Biol. Biochem. 2016, 103, 489–492. [Google Scholar] [CrossRef]
- Mainiero, R.; Kazda, M. Depth-related fine root dynamics of Fagus sylvatica during exceptional drought. For. Ecol. Manag. 2006, 237, 135–142. [Google Scholar] [CrossRef]
- Teskey, R.O.; Hinckley, T.M. Influence of temperature and water potential on root growth of white oak. Physiol. Plant. 1981, 52, 363–369. [Google Scholar] [CrossRef]
- López, B.; Sabaté, S.; Gracia, C. Fine roots dynamics in a Mediterranean forest: Effects of drought and stem density. Tree Physiol. 1998, 18, 601–606. [Google Scholar] [CrossRef] [Green Version]
- Thongo M’bou, A.; Jourdan, C.; Deleporte, P.; Nouvellon, Y.; Saint-André, L.; Bouillet, J.P.; Mialoundama, F.; Mabiala, A.; Epron, D. Root elongation in tropical Eucalyptus plantations: Effect of soil water content. Ann. For. Sci. 2008, 65, 609. [Google Scholar] [CrossRef] [Green Version]
- Laclau, J.P.; Arnaud, M.; Bouillet, J.P.; Ranger, J. Spatial distribution of Eucalyptus roots in a deep sandy soil in the Congo: Relationships with the ability of the stand to take up water and nutrients. Tree Physiol. 2001, 21, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Mu, X.; Yuan, Z.; Deng, Q.; Chen, Y.; Yuan, L.Y.; Ryan, L.T.; Kallenbach, R.L. Soil nutrients and water affect the age-related fine root biomass but not production in two plantation forests on the Loess Plateau, China. J. Arid. Environ. 2016, 135, 173–180. [Google Scholar] [CrossRef]
- Espeleta, J.F.; Clark, D.A. Multi-scale variation in fine-root biomass in a tropical rain forest: A seven-year study. Ecol. Monogr. 2007, 77, 377–404. [Google Scholar] [CrossRef] [Green Version]
- Ceccon, C.; Panzacchi, P.; Scandellari, F.; Prandi, L.; Ventura, M.; Russo, B.; Millard, P.; Tagliavini, M. Spatial and temporal effects of soil temperature and moisture and the relation to fine root density on root and soil respiration in a mature apple orchard. Plant Soil 2011, 342, 195–206. [Google Scholar] [CrossRef]
- Bakker, M.R.; Augusto, L.; Achat, D.L. Fine root distribution of trees and understory in mature stands of maritime pine (Pinus pinaster) on dry and humid sites. Plant Soil 2006, 286, 37–51. [Google Scholar] [CrossRef]
- Japan Forestry Agency. Annual Report on Forest and Forestry in Japan Fiscal Year2016; Summary; Ministry of Agriculture, Forestry and Fisheries: Japan, Tokyo, 2017; p. 30. Available online: http://www.rinya.maff.go.jp/j/kikaku/hakusyo/28hakusyo/attach/pdf/index-1.pdf (accessed on 25 May 2018).
- Karizumi, N. The mechanism and function of tree root in the process of forest production. I. Method of investigation and estimation of the root biomass. Bull. Gov. For. Exp. Stn. 1974, 259, 1–99. [Google Scholar]
- Fujimaki, R.; Tateno, R.; Tokuchi, N. Root development across a chronosequence in a Japanese cedar (Cryptomeria japonica D. Don) plantation. J. For. Res. 2007, 12, 96–102. [Google Scholar] [CrossRef]
- Farahnak, M.; Mitsuyasu, K.; Jeong, S.; Otsuki, K.; Chiwa, M.; Sadeghi, S.M.M.; Kume, A. Soil hydraulic conductivity differences between upslope and downslope of two coniferous trees on a hillslope. J. For. Res. 2019, 24, 143–152. [Google Scholar] [CrossRef]
- Komatsu, H.; Shinohara, Y.; Otsuki, K. Models to predict changes in annual runoff with thinning and clear-cutting of Japanese cedar and cypress plantations in Japan. Hydrol. Process. 2015, 29, 5120–5134. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Onda, Y.; Kato, H.; Gomi, T.; Liu, X. Estimation of throughfall with changing stand structures for Japanese cypress and cedar plantations. For. Ecol. Manag. 2017, 402, 145–156. [Google Scholar] [CrossRef]
- Jeong, S.; Otsuki, K.; Farahnak, M. Relationship between stand structures and rainfall partitioning in dense unmanaged Japanese cypress plantations. J. Agric. Meteorol. 2019, 75, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.; Otsuki, K.; Shinohara, Y.; Inoue, A.; Ichihashi, R. Stemflow estimation models for Japanese cedar and cypress plantations using common forest inventory data. Agric. For. Meteorol. 2020, 290, 107997. [Google Scholar] [CrossRef]
- Farahnak, M.; Mitsuyasu, K.; Otsuki, K.; Shimizu, K.; Kume, A. Factors determining soil water repellency in two coniferous plantations on a hillslope. Forests 2019, 10, 730. [Google Scholar] [CrossRef] [Green Version]
- Kuraji, K. Effects of Forests on Stabilizing Streamflow; Nihon Chisan-chisui Kyokai: Tokyo, Japan, 2003; p. 76. (In Japanese) [Google Scholar]
- Onda, Y.; Gomi, T.; Mizugaki, S.; Nonoda, T.; Sidle, R.C. An overview of the field and modelling studies on the effects of forest devastation on flooding and environmental issues. Hydrol. Process. 2010, 2274, 2267–2274. [Google Scholar] [CrossRef]
- Ganatsios, H.P.; Tsioras, P.A.; Pavlidis, T. Water yield changes as a result of silvicultural treatments in an oak ecosystem. For. Ecol. Manag. 2010, 260, 1367–1374. [Google Scholar] [CrossRef]
- Dung, B.X.; Miyata, S.; Gomi, T. Effect of forest thinning on overland flow generation on hillslopes covered by Japanese cypress. Ecohydrology 2011, 4, 367–378. [Google Scholar] [CrossRef]
- Shinohara, Y.; Levia, D.F.; Komatsu, H.; Nogata, M.; Otsuki, K. Comparative modeling of the effects of intensive thinning on canopy interception loss in a Japanese cedar (Cryptomeria japonica D. Don) forest of western Japan. Agric. For. Meteorol. 2015, 214, 148–156. [Google Scholar] [CrossRef]
- Hishi, T.; Takeda, H. Dynamics of heterorhizic root systems: Protoxylem groups within the fine-root system of Chamaecyparis obtusa. New Phytol. 2005, 167, 509–521. [Google Scholar] [CrossRef]
- Hishi, T.; Takeda, H. Life cycles of individual roots in fine root system of Chamaecyparis obtusa Sieb. et Zucc. J. For Res. 2005, 10, 181–187. [Google Scholar] [CrossRef]
- Forest Soil Division. Classification of forest soil in Japan (1975). Bull. Gov. For. Exp. Sta 1976, 280, 1–28, (In Japanese with English summary). [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, update 2015: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Res. Rep. 2015, 106, 192. [Google Scholar]
- Geotechnical Society and Guidance Soil Test Basic. 2010. (In Japanese). Available online: https://catalog.lib.kyushu-u.ac.jp/en/recordID/1001422498/ (accessed on 23 March 2018).
- Konôpka, B.; Noguchi, K.; Sakata, T.; Takahashi, M.; Konôpková, Z. Fine root dynamics in a Japanese cedar (Cryptomeria japonica) plantation throughout the growing season. For. Ecol. Manag. 2006, 225, 278–286. [Google Scholar] [CrossRef]
- Konôpka, B.; Noguchi, K.; Sakata, T.; Takahashi, M.; Konôpková, Z. Effects of simulated drought stress on the fine roots of Japanese cedar (Cryptomeria japonica) in a plantation forest on the Kanto Plain, eastern Japan. J. For. Res. 2007, 12, 143–151. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Persson, H.Å. The distribution and productivity of fine roots in boreal forests. Plant Soil 1983, 71, 87–101. [Google Scholar] [CrossRef]
- Weemstra, M.; Mommer, L.; Visser, E.J.; van Ruijven, J.; Kuyper, T.W.; Mohren, G.M.; Sterck, F.J. Towards a multidimensional root trait framework: A tree root review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef] [Green Version]
- Ford, E.D.; Deans, J.D. Growth of a sitka spruce plantation: Spatial distribution and seasonal fluctuations of lengths, weights and carbohydrate concentrations of fine roots. Plant Soil 1977, 47, 463–485. [Google Scholar] [CrossRef]
- Raizada, A.; Jayaprakash, J.; Rathore, A.C.; Tomar, J.M.S. Distribution of fine root biomass of fruit and forest tree species raised on old river bed lands in the North West Himalaya. Trop. Ecol. 2013, 54, 251–261. [Google Scholar]
- Zhang, Y.; Niu, J.; Yu, X.; Zhu, W.; Du, X. Effects of fine root length density and root biomass on soil preferential flow in forest ecosystems. For. Syst. 2015, 24, 12. [Google Scholar] [CrossRef] [Green Version]
- Fujii, S.; Kasuya, N. Fine root biomass and morphology of Pinus densiflora under competitive stress by Chamaecyparis obtusa. J. For. Res. 2008, 13, 185–189. [Google Scholar] [CrossRef]
- Miyatani, K.; Mizusawa, Y.; Okada, K.; Tanikawa, T.; Makita, N.; Hirano, Y. Fine root traits in Chamaecyparis obtusa forest soils with different acid buffering capacities. Trees 2016, 30, 415–429. [Google Scholar] [CrossRef]
- Sawata, S.; Kato, H. Effect of forest on soil (part 2). The base accumulation and other soil properties related to age of Cryptomeria and Japanese cypress stands. Jpn. J. Soil Sci. Plant Nutr. 1991, 62, 49–58, (In Japanese with English abstract). [Google Scholar]
- Ohta, T.; Niwa, S.; Hiura, T. Calcium concentration in leaf litter affects the abundance and survival of crustaceans in streams draining warm–temperate forests. Freshw. Biol. 2014, 59, 748–760. [Google Scholar] [CrossRef]
- Tanikawa, T.; Ito, Y.; Fukushima, S.; Yamashita, M.; Sugiyama, A.; Mizoguchi, T.; Okamoto, T.; Hirano, Y. Calcium is cycled tightly in Cryptomeria japonica stands on soils with low acid buffering capacity. For. Ecol. Manag. 2017, 399, 64–73. [Google Scholar] [CrossRef]
- Hirano, Y.; Tanikawa, T.; Makita, N. Biomass and morphology of fine roots in eight Cryptomeria japonica stands in soils with different acid-buffering capacities. For. Ecol. Manag. 2017, 384, 122–131. [Google Scholar] [CrossRef]
- Gower, S.T. Relations between mineral nutrient availability and fine root biomass in two Costa Rican tropical wet forests: A hypothesis. Biotropica 1987, 171–175. [Google Scholar] [CrossRef]
- DeBano, L.F. Water repellency in soils: A historical overview. J. Hydrol. 2000, 231, 4–32. [Google Scholar] [CrossRef]
- Cerdà, A.; Doerr, S.H. Soil wettability, runoff and erodibility of major dry-Mediterranean land use types on calcareous soils. Hydrol. Process. 2007, 21, 2325–2336. [Google Scholar] [CrossRef]
- Leung, A.K.; Garg, A.; Coo, J.L.; Ng, C.W.W.; Hau, B.C.H. Effects of the roots of Cynodon dactylon and Schefflera heptaphylla on water infiltration rate and soil hydraulic conductivity. Hydrol. Process. 2015, 29, 3342–3354. [Google Scholar] [CrossRef] [Green Version]





| Tree Species | Cryptomeria Japonica | Chamaecyparis Obtusa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plots | Intact Tree | Cut Tree | Intact Tree | Cut Tree | ||||||||
| Soil depth (cm) | 0–5 | 5–10 | 10–30 | 0–5 | 5–10 | 10–30 | 0–5 | 5–10 | 10–30 | 0–5 | 5–10 | 10–30 |
| Sand (%) | 63.13 | 61.11 | 47.56 | 70.03 | 60.92 | 55.78 | 75.14 | 59.54 | 61.47 | 73.08 | 65.61 | 55.96 |
| Clay (%) | 10.94 | 13.26 | 25.53 | 9.72 | 14.41 | 15.72 | 9.28 | 15.17 | 12.60 | 11.78 | 13.00 | 15.92 |
| Soil bulk density | 1.06 | 1.09 | 1.23 | 0.87 | 1.05 | 1.13 | 0.91 | 1.04 | 1.18 | 0.86 | 1.02 | 1.15 |
| Soil organic matter | 12.99 | 9.21 | 8.11 | 11.66 | 8.86 | 7.29 | 13.07 | 8.59 | 7.42 | 14.22 | 10.21 | 8.00 |
| Ca2+ | 11.08 | 5.92 | 3.07 | 14.51 | 9.61 | 5.97 | 2.87 | 2.40 | 3.20 | 5.35 | 5.19 | 4.96 |
| Soil pH (KCl) | 4.03 | 3.92 | 3.80 | 4.30 | 4.08 | 3.87 | 3.47 | 3.59 | 3.74 | 3.71 | 3.82 | 3.95 |
| Soil water repellency * | 1.80 | 0.0 | 0.0 | 3.05 | 0.0 | 0.0 | 13.10 | 3.60 | 0.0 | 11.65 | 3.50 | 0.0 |
| Soil hydraulic conductivity | 9.59 | – | – | 13.51 | – | – | 2.17 | – | – | 2.75 | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farahnak, M.; Mitsuyasu, K.; Hishi, T.; Katayama, A.; Chiwa, M.; Jeong, S.; Otsuki, K.; Sadeghi, S.M.M.; Kume, A. Relationship between Very Fine Root Distribution and Soil Water Content in Pre- and Post-Harvest Areas of Two Coniferous Tree Species. Forests 2020, 11, 1227. https://doi.org/10.3390/f11111227
Farahnak M, Mitsuyasu K, Hishi T, Katayama A, Chiwa M, Jeong S, Otsuki K, Sadeghi SMM, Kume A. Relationship between Very Fine Root Distribution and Soil Water Content in Pre- and Post-Harvest Areas of Two Coniferous Tree Species. Forests. 2020; 11(11):1227. https://doi.org/10.3390/f11111227
Chicago/Turabian StyleFarahnak, Moein, Keiji Mitsuyasu, Takuo Hishi, Ayumi Katayama, Masaaki Chiwa, Seonghun Jeong, Kyoichi Otsuki, Seyed Mohammad Moein Sadeghi, and Atsushi Kume. 2020. "Relationship between Very Fine Root Distribution and Soil Water Content in Pre- and Post-Harvest Areas of Two Coniferous Tree Species" Forests 11, no. 11: 1227. https://doi.org/10.3390/f11111227