Abstract
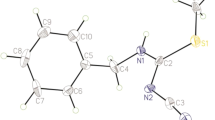
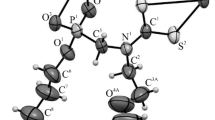
Diazo-transfer reaction of tosyl azide and alkyl 2-cyanoacetates in the presence of pyridine at 0 °C gives the corresponding alkyl 2-cyano-2-diazoacetates. This material readily undergoes nucleophilic attack by pyridinium 1-cyano-2-alkoxy-2-oxoethan-1-ide to form dialkyl (Z)-3-amino-2-cyano-4-diazopent-2-enedioates. The X-ray structure of a typical product is reported, as well as DFT computational studies that illuminate the structural features of these stable diazo compounds.
Graphic abstract





Similar content being viewed by others
References
Marinozzi M, Pertusati F, Serpi M (2016) Chem Rev 116:13991
Liu L, Zhang J (2016) Chem Soc Rev 45:506
Xu X, Doyle MP (2014) Acc Chem Res 47:1396
Guo X, Hu W (2013) Acc Chem Res 46:2427
Davies HM, Morton D (2011) Chem Soc Rev 40:1857
Curtius T (1883) Ber Dtsch Chem Ges 16:2230
Chiles HM, Noyes WA (1922) J Am Chem Soc 44:1798
Maas G (2009) Angew Chem Int Ed 48:8186
Johnston JN, Muchalski H, Troyer TL (2010) Angew Chem Int Ed 49:2290
Ye T, McKervey MA (1994) Chem Rev 94:1091
Davies HM, Manning JR (2008) Nature 451:417
Xia Y, Wang J (2017) Chem Soc Rev 46:2306
Wan JP, Cao S, Liu Y (2016) Org Lett 18:6034
Li AH, Dai LX, Aggarwal VK (1997) Chem Rev 97:2341
Wang J (2010) J Am Chem Soc 132:13590
Gan L, Wei L, Wan JP (2020) ChemistrySelect 5:7822
Zhang Z, Wang J (2008) Tetrahedron 64:6577
Chow S, Green A, Arter C, Liver S, Leggott A, Trask L, Karageorgis G, Warriner S, Nelson A (2020) Synthesis 52:1695
Xiangyan Y, Zhipeng Z, He H, Jonathan B, Yang Y, Fei F (2019) Chin J Org Chem 39:544
He HY, Ding WD, Bernan VS, Richardson AD, Ireland CM, Greenstein M, Ellestad GA, Carter GT (2001) J Am Chem Soc 123:5362
Köpke T, Zaleski JM (2008) Anti-Cancer Agents Med Chem 8:292
Nawrat CC, Moody CJ (2011) Nat Prod Rep 28:1426
Bartz QR, Elder CC, Frohardt RP, Fusari SA, Haskell TH, Johannessen DW, Ryder A (1954) Nature 173:72
Woo CM, Gholap SL, Lu MK, Kaneko M, Li Z, Ravikumar PC, Herzon SB (2012) J Am Chem Soc 134:17262
Nicolaou KC, Li H, Nold AL, Pappo D, Lenzen A (2007) J Am Chem Soc 129:10356
Mix KA, Aronoff MR, Raines RT (2016) ACS Chem Biol 11:3233
Deng L, Cao X, Liu Y, Wan JP (2019) J Org Chem 84:14179
Filimonov VO, Dianova LN, Beryozkina TV, Mazur D, Beliaev NA, Volkova NN, Ilkin VG, Dehaen W, Lebedev AT, Bakulev VA (2019) J Org Chem 84:13430
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich A, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JAJ, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam J, Klene MM, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 09, Revision A02. Gaussian Inc, Wallingford
Sustmann R (1974) Pure Appl Chem 40:569
Costa J, Taveira R, Lima C, Mendes A, Santos L (2016) Opt Mater 58:51
Acknowledgements
We would like to thank the Research Council of Tarbiat Modares University for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yavari, I., Mohsenzadeh, R. & Kayanian, J. Synthesis and structure of dialkyl (Z)-3-amino-2-cyano-4-diazopent-2-enedioates. Monatsh Chem 151, 1829–1834 (2020). https://doi.org/10.1007/s00706-020-02712-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-020-02712-4