Abstract
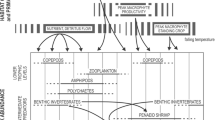
Mesopelagic fishes are important consumers of zooplankton and are the prey of oceanic predators. Some mesopelagic fishes (e.g., Myctophidae) undertake a diel vertical migration where they ascend to the near-surface waters during the night to feed and descend into the depths during the day to avoid predators. Other mesopelagic fishes (e.g., Sternoptyx) remain at depth throughout the day. Although fishes of different depths eat different prey items, vertical migration likely leads to overlap in species distributions and diet, potentially linking trophically transmitted surface and deep-pelagic parasite communities. The study of gut contents and parasites can yield insights into the trophic dynamics occurring within these assemblages. We examined the diet and parasite assemblages of 18 mesopelagic fish species in the Gulf of Mexico. We identified six different feeding guilds within this assemblage based on gut contents: copepods, copepods/mesozooplankton, copepods/ostracods, gelatinous zooplankton, generalist mesozooplankton, and upper-trophic level items such as fish. Although parasite abundances were generally low, mesopelagic fishes hosted a diverse assemblage of parasites, including larval and adult digeneans, larval cestodes, larval nematodes, and larval acanthocephalans. The parasite assemblages differed significantly among host feeding guilds. Large, vertically migrating fishes that predate upon fish and squid communities had a greater likelihood of having one or more parasites compared to the other fishes examined. Results from this study suggest that upper-trophic level mesopelagic fishes are more regularly involved in the life cycle of parasites in the Gulf of Mexico than zooplanktivorous fishes.



Similar content being viewed by others
Availability of data and material
Data are publicly available through the Gulf of Mexico Research Initiative Information and Data Cooperative (GRIIDC) at https://data.gulfresearchinitiative.org (https://doi.org/10.7266/N7XP73851; https://doi.org/10.7266/N7902234).
References
Amin OM (1998) Marine flora and fauna of the eastern United States: Acanthocephala. NOAA Technical Report NMFS 135
Andres MJ, Peterson MS, Overstreet RM (2016) Endohelminth parasites of some midwater and benthopelagic stomiiform fishes from the northern Gulf of Mexico. Gulf Carib Res 27:11–19. https://doi.org/10.18785/gcr.2701.02
Blend CK, Dronen NO, Franks JS, Benz GW (2010) Endohelminths of a snake mackerel, Gempylus serpens (Trichiuroidea: Gempylidae), from the Gulf of Mexico. Gulf Carib Res 22:1–8
Boxshall GA, Halsey SH (2004) An introduction to copepod diversity. Ray Society, Andover
Bozdogan H (1987) Model selection and Akaike’s Information Criterion (AIC): the general theory and its analytical extensions. Psychometrika 52:345–370
Bray RA (2020) Digenean parasites of deep-sea teleosts: a progress report. Int J Parasitol Parasites Wildl. https://doi.org/10.1016/j.ijppaw.2020.01.007
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349. https://doi.org/10.2307/1942268
Bray RA, Littlewood DTJ, Herniou EA, Williams B, Henderson RE (1999) Digenean parasites of deep-sea teleosts: a review and case studies of intrageneric phylogenies. Parasitology 119(Suppl.):S125–S144
Bray RA, Gibson DI, Jones A (2008) Keys to the Trematoda, vol 3. CABI Publishing and The Natural History Museum, Wallingford
Busch MW, Klimpel S, Sutton T, Piatkowski U (2008) Parasites of the deep-sea smelt Bathylagus euryops (Argentiniformes: Microstomatidae) from the Charlie-Gibbs Fracture Zone (CGFZ). Mar Biol Res 4:313–317. https://doi.org/10.1080/17451000801907963
Clarke KR, Somerfield PJ, Chapman MG (2006) On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray-Curtis coefficient for denuded assemblages. J Exp Mar Biol and Ecol 330:55–80. https://doi.org/10.1016/j.jembe.2005.12.017
Cribb TH (2005) Family Opecoelidae Ozaki, 1925. In: Jones A, Bray RA, Gibson DI (eds) Keys to the Trematoda, vol 2. CABI Publishing and The Natural History Museum, Wallingford, pp 443–531
Gartner JV, Zwerner DE (1989) The parasite faunas of meso-and bathypelagic fishes of Norfolk Submarine Canyon, western North Atlantic. J Fish Biol 34:79–95. https://doi.org/10.1111/j.1095-8649.1989.tb02959.x
Gibson DI, Jones A, Bray RA (2002) Keys to the Trematoda, vol 1. CABI Publishing and The Natural History Museum, Wallingford
Hawes S, Miskiewicz T, Garcia V, Figueira W (2020) Size and stage-dependent vertical migration patterns in reef-associated fish larvae off the eastern coast of Australia. Deep Sea Res Part I Oceanogr Res Pap 162:103362. https://doi.org/10.1016/j.dsr.2020.103362
Hoberg EP (1996) Faunal diversity among avian parasite assemblages: the interaction of history, ecology, and biogeography in marine systems. Bull Scand Soc Parasitol 6:65–89
Hopkins TL, Baird RC (1985) Feeding ecology of four hatchetfishes (Sternoptychidae) in the eastern Gulf of Mexico. Bull Mar Sci 36:260–277
Hopkins TL, Gartner JV (1992) Resource partitioning and predation impact of a low-latitude myctophid community. Mar Biol 114:185–197. https://doi.org/10.1007/BF00349518
Hopkins TL, Sutton TT (1998) Midwater fishes and shrimps as competitors and resource partitioning in low latitude oligotrophic ecosystems. Mar Ecol Prog Ser 164:37–45. https://doi.org/10.3354/meps164037
Hopkins TL, Sutton TT, Lancraft TM (1996) The trophic structure and predation impact of a low latitude midwater fish assemblage. Prog Oceanogr 38:205–239. https://doi.org/10.1016/S0079-6611(97)00003-7
Jones A, Bray RA, Gibson DI (2005) Keys to the Trematoda, vol 2. CABI Publishing and The Natural History Museum, Wallingford
Khalil LF, Jones A, Bray RA (1994) Keys to the cestode parasites of vertebrates. CABI Publishing and The Natural History Museum, Wallingford
Klimpel S, Seehagen A, Palm HW, Rosenthal H (2001) Deep-water metazoan fish parasites of the world. Logos Verlag, Berlin
Klimpel S, Palm HW, Busch MW, Kellermanns E, Rückert S (2006) Fish parasites in the Arctic deep-sea: poor diversity in pelagic fish species vs. heavy parasite load in a demersal fish. Deep Sea Res Part I Oceanogr Res Pap 53:1167–1181. https://doi.org/10.1016/j.dsr.2006.05.009
Lafferty KD, Dobson AP, Kuris AM (2006) Parasites dominate food web links. Proc Natl Acad Sci 103:11211–11216. https://doi.org/10.1073/pnas.0604755103
MacKenzie K, Abaunza P (1998) Parasites as biological tags for stock discrimination of marine fish: a guide to procedures and methods. Fish Res 38:45–56. https://doi.org/10.1016/S0165-7836(98)00116-7
Marcogliese DJ (2002) Food webs and the transmission of parasites to marine fish. Parasitology 124:83–99. https://doi.org/10.1017/S003118200200149X
McClain-Counts JP, Demopoulos AWJ, Ross SW (2017) Trophic structure of mesopelagic fishes in the Gulf of Mexico revealed by gut content and stable isotope analyses. Mar Ecology 38:e12449. https://doi.org/10.1111/maec.12449
Noble ER, Orias JD (1975) Parasitism in the bathypelagic fish, Melanostigma pammelas. Int J Parasitol 5:89–93. https://doi.org/10.1016/0020-7519(75)90103-4
Oksanen, J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’hara RR, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2019) vegan: Community Ecology Package. R package version 2.5–6. https://CRAN.R-project.org/package=vegan. Accessed 17 Nov 2019
Pritchard MH, Kruse GO (1982) The collection and preservation of animal parasites. University of Nebraska Press, Lincoln
Richards TM, Gipson EE, Cook A, Sutton TT, Wells RJ (2018) Trophic ecology of meso- and bathypelagic predatory fishes in the Gulf of Mexico. ICES J Mar Sci 76:662–672. https://doi.org/10.1093/icesjms/fsy074
Schmidt GD (1986) CRC handbook of tapeworm identification. CRC Press, Inc., Boca Raton
Sutton TT, Hopkins TL (1996) Trophic ecology of the stomiid (Pisces: Stomiidae) fish assemblage of the eastern Gulf of Mexico: strategies, selectivity, and impact of a top mesopelagic predator group. Mar Biol 127:179–192. https://doi.org/10.1007/BF00942102
Sutton TT, Mercier P (2012) NRDA Nekton processing plan attachment, vol 2. Trawl Volume Calculations. Unpublished report
Sutton TT, Clark MR, Dunn DC, Halpin PN, Rogers AD, Guinotte J, Bograd SJ, Angel MV, Perez JAA, Wishner K, Haedrich RL, Lindsay DJ, Drazen JC, Vereshchaka A, Piatkowski U, Morato T, Błachowiak-Samołyk K, Robison BH, Gjerde KM, Pierrot-Bults A, Bernal P, Reygondeau G, Heino M (2017) A global biogeographic classification of the mesopelagic zone. Deep Sea Res I 126:85–102. https://doi.org/10.1016/j.dsr.2017.05.006
Sutton TT, Frank TM, Romero IC, Judkins H (2020) Chapter 24: as Gulf oil extraction goes deeper, who is at risk? Community structure, distribution, and connectivity of the deep-pelagic fauna. In: Murawski SA, Ainsworth C, Gilbert S, Hollander D, Paris CB, Schlüter M, Wetzel D (eds) Scenarios and responses to future deep oil spills—fighting the next war. Springer, Cham, pp 403–418
Webb TJ, Berghe EV, O’Dor R (2010) Biodiversity’s big wet secret: the global distribution of marine biological records reveals chronic under-exploration of the deep pelagic ocean. PLoS ONE 5:10223. https://doi.org/10.1371/journal.pone.0010223
Wiebe PH, Morton AW, Bradley AM, Backus RH, Craddock JE, Barber V, Cowles TJ, Flierl GD (1985) New development in the MOCNESS, an apparatus for sampling zooplankton and micronekton. Mar Biol 87:313–323. https://doi.org/10.1007/BF00397811
Woodstock MS (2018) Trophic ecology and parasitism of a mesopelagic fish assemblage. Nova Southeastern University, Thesis
Zubchenko AV (1981) Parasitic fauna of some Macrouridae in the Northwest Atlantic. J Northwest Atl Fish Sci 2:67–72
Acknowledgements
The authors are grateful to Dr. Rosanna Milligan for her help with linear modeling and two anonymous reviewers who improved earlier versions of this manuscript. This research was made possible in part by a grant from The Gulf of Mexico Research Initiative. Data are publicly available through the Gulf of Mexico Research Initiative Information & Data Cooperative (GRIIDC) at https://data.gulfresearchinitiative.org (https://doi.org/10.7266/N7XP73851; https://doi.org/10.7266/N7902234).
Funding
This research was made possible in part by a grant from The Gulf of Mexico Research Initiative.
Author information
Authors and Affiliations
Contributions
All authors were present at the development of the project, TTS and MSW gathered fish samples, MSW and CAB identified parasites, MSW and TTS identified prey contents, MSW wrote the initial manuscript, All authors revised and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Specimens for this study were collected during a long-standing collaboration between one of the authors [TTS] and Dr. Jon Moore at Florida Atlantic University. Dr. Moore held the IACUC permit for all research cruises conducted by the DEEPEND consortium, of which TTS is the Director/Principal Investigator.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability (software application or custom code)
R code for figures and applicable datasets will be made available at the time of publication at https://github.com/mwood078/mesopelagic_parasites_gom.
Additional information
Responsible Editor: T. Reusch.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by M. Andres and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Woodstock, M.S., Blanar, C.A. & Sutton, T.T. Diet and parasites of a mesopelagic fish assemblage in the Gulf of Mexico. Mar Biol 167, 184 (2020). https://doi.org/10.1007/s00227-020-03796-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-020-03796-6