Abstract
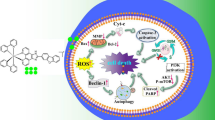
Ruthenium complexes have been recently reported as potential chemotherapeutic agents that offer tumor selectivity and low tumor resistance. This study investigates the photochemistry and the effect of four strained photoactivatable polypyridyl ruthenium(II) complexes on non-small-cell lung cancer (A549) and triple negative breast cancer (MDA-MB-231) cells. All four ruthenium(II) complexes, [Ru(bpy)2dmbpy]Cl2 (C1) where (bpy = 2,2′-bipyridine and dmbpy = 6,6′-dimethyl-2,2′-bipyridine), [Ru(phen)2dmbpy]Cl2 (C2) where (phen = 1,10-phenanthroline), [Ru(dpphen)2dmbpy]Cl2 (C3) (where dpphen = 4,7-diphenyl-1,10-phenanthroline) and [Ru(BPS)2dmbpy]Na2 (C4) where (BPS = bathophenanthroline disulfonate) eject the dmbpy ligand upon activation by blue light. Determination of the octanol–water partition coefficient (log P) revealed that C3 was the only lipophilic complex (log P = 0.42). LC–MS/MS studies showed that C3 presented the highest cellular uptake. The cytotoxic effect of the complexes was evaluated with and without blue light activation using WST-1 kit. Data indicated that C3 exhibited the highest cytotoxicity after 72 h (MDA-MB-231, IC50 = 0.73 µM; A549, IC50 = 1.26 µM) of treatment. The phototoxicity indices of C3 were 6.56 and 4.64 for MDA-MB-230 and A549, respectively. Upon light activation, C3 caused significant ROS production and induced apoptosis in MDA-MB-231 cells as shown by flow cytometry. It also significantly increased Bax/Bcl2 ratio and PERK levels without affecting caspase-3 expression. C3 exhibited poor dark toxicity (IC50 = 74 μM) on rat mesenchymal stem cells (MSCs). In conclusion, the physical property of the complexes dictated by the variable ancillary ligands influenced cellular uptake and cytotoxicity. C3 may be considered a promising selective photoactivatable chemotherapeutic agent that induces ROS production and apoptosis.








Similar content being viewed by others
References
Desoize B (2004) Metals and metal compounds in cancer treatment. Anticancer Res 24(3a):1529–1544
Knoll JD, Turro C (2015) Control and utilization of ruthenium and rhodium metal complex excited states for photoactivated cancer therapy. Coord Chem Rev 282–283:110–126. https://doi.org/10.1016/j.ccr.2014.05.018
Ho GY, Woodward N, Coward JIG (2016) Cisplatin versus carboplatin: comparative review of therapeutic management in solid malignancies. Crit Rev Oncol Hematol 102:37–46. https://doi.org/10.1016/j.critrevonc.2016.03.014
Kelland L (2007) The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 7(8):573–584. https://doi.org/10.1038/nrc2167
Ott I, Gust R (2007) Non platinum metal complexes as anti-cancer drugs. Arch Pharm (Weinheim) 340(3):117–126. https://doi.org/10.1002/ardp.200600151
Guo W et al (2013) Transferrin serves as a mediator to deliver organometallic ruthenium(II) anticancer complexes into cells. Inorg Chem 52(9):5328–5338. https://doi.org/10.1021/ic4002626
Schluga P et al (2006) Redox behavior of tumor-inhibiting ruthenium(iii) complexes and effects of physiological reductants on their binding to GMP. Dalton Trans Camb Engl. https://doi.org/10.1039/b511792e
Mari C, Pierroz V, Ferrari S, Gasser G (2015) Combination of Ru(ii) complexes and light: new frontiers in cancer therapy. Chem Sci 6(5):2660–2686. https://doi.org/10.1039/C4SC03759F
Fandzloch M, Dobrzańska L, Jędrzejewski T, Jezierska J, Wiśniewska J, Łakomska I (2019) Synthesis, structure and biological evaluation of ruthenium(III) complexes of triazolopyrimidines with anticancer properties. J Biol Inorg Chem 25(1):109–124. https://doi.org/10.1007/s00775-019-01743-5
Stacey OJ, Pope SJA (2013) New avenues in the design and potential application of metal complexes for photodynamic therapy. RSC Adv 3(48):25550. https://doi.org/10.1039/c3ra45219k
Arenas Y, Monro S, Shi G, Mandel A, McFarland S, Lilge L (2013) Photodynamic inactivation of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus with Ru(II)-based type I/type II photosensitizers. Photodiagnosis Photodyn Ther 10(4):615–625. https://doi.org/10.1016/j.pdpdt.2013.07.001
Park W, Bae B, Na K (2016) A highly tumor-specific light-triggerable drug carrier responds to hypoxic tumor conditions for effective tumor treatment. Biomaterials 77:227–234. https://doi.org/10.1016/j.biomaterials.2015.11.014
Bonnet S (2018) Why develop photoactivated chemotherapy? Dalton Trans 47(31):10330–10343. https://doi.org/10.1039/C8DT01585F
Farrer NJ, Salassa L, Sadler PJ (2009) Photoactivated chemotherapy (PACT): the potential of excited-state d-block metals in medicine. Dalton Trans Camb Engl. https://doi.org/10.1039/b917753a
Mahnken RE, Billadeau MA, Nikonowicz EP, Morrison H (1992) Development of photo cis-platinum reagents. Reaction of cis-dichlorobis(1,10-phenanthroline)rhodium(III) with calf thymus DNA, nucleotides and nucleosides. J Am Chem Soc 114(24):9253–9265. https://doi.org/10.1021/ja00050a002
Durham B, Caspar JV, Nagle JK, Meyer TJ (1982) Photochemistry of tris(2,2’-bipyridine)ruthenium(2+) ion. J Am Chem Soc 104(18):4803–4810. https://doi.org/10.1021/ja00382a012
Campagna S, Puntoriero F, Nastasi F, Bergamini G, Balzani V (2007) Photochemistry and photophysics of coordination compounds: ruthenium. In: Balzani V, Campagna S (eds) Photochemistry and photoph. Springer, Berlin, pp 117–214
Howerton BS, Heidary DK, Glazer EC (2012) Strained ruthenium complexes are potent light-activated anticancer agents. J Am Chem Soc 134(20):8324–8327. https://doi.org/10.1021/ja3009677
Juris A, Balzani V, Barigelletti F, Campagna S, Belser P, von Zelewsky A (1988) Ru(II) polypyridine complexes: photophysics, photochemistry, eletrochemistry, and chemiluminescence. Coord Chem Rev 84:85–277. https://doi.org/10.1016/0010-8545(88)80032-8
Laemmel A-C, Collin J-P, Sauvage J-P (1999) Efficient and selective photochemical labilization of a given bidentate ligand in mixed ruthenium(II) complexes of the Ru(phen)2L2+ and Ru(bipy)2L2+ family (L = sterically hindering chelate). Eur J Inorg Chem 1999(3):383–386. https://doi.org/10.1002/(SICI)1099-0682(199903)1999:3%3c383::AID-EJIC383%3e3.0.CO;2-9
Mehanna S et al (2019) Enhanced cellular uptake and photochemotherapeutic potential of a lipophilic strained Ru(ii) polypyridyl complex. RSC Adv 9(30):17254–17265. https://doi.org/10.1039/C9RA02615K
Mansour N et al (2018) Photoactivatable ruii complex bearing 2,9-diphenyl-1,10-phenanthroline: unusual photochemistry and significant potency on cisplatin-resistant cell lines. Eur J Inorg Chem 2018(22):2524–2532. https://doi.org/10.1002/ejic.201800194
Liu C, Yu L, Liu Y, Li F, Zhou M (2011) NMR study on iridium(III) complexes for identifying disulfonate substituted bathophenanthroline regio-isomers. Magn Reson Chem 49(12):816–823. https://doi.org/10.1002/mrc.2815
Della Ciana L, Zanarini S, Perciaccante R, Marzocchi E, Valenti G (2010) Neutral and dianionic Ru(II) bathophenanthrolinedisulfonate complexes: a route to enhance electrochemiluminescence performance in aqueous media. J Phys Chem C 114(8):3653–3658. https://doi.org/10.1021/jp910596z
Hackett JW, Turro C (1998) Luminescent Ru(phen)n(bps)3–n2n-4 complexes (n = 0–3) as probes of electrostatic and hydrophobic interactions with micellar media. Inorg Chem 37(8):2039–2046. https://doi.org/10.1021/ic970801b
Cuello-Garibo J, Meijer MS, Bonnet S (2017) To cage or to be caged? The cytotoxic species in ruthenium-based photoactivated chemotherapy is not always the metal. Chem Commun 53(50):6768–6771. https://doi.org/10.1039/C7CC03469E
Al Hageh C et al (2018) A long-lived cuprous bis-phenanthroline complex for the photodynamic therapy of cancer. Dalton Trans Camb Engl 47(14):4959–4967. https://doi.org/10.1039/C8DT00140E
Mehanna S, Bodman-Smith K, Daher CF, Khnayzer RS (2020) Rapid quantification of ruthenium(ii) polypyridyl anti-cancer drugs using a selective ligand dissociation LC-MS/MS method. Anal Methods. https://doi.org/10.1039/D0AY01250E
Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies, & National Research Council (2011) Guide for the care and use of laboratory animals (8th edn). National Academies Press
Smajilagić A, Aljičević M, Redžić A, Filipović S, Lagumdžija A (2013) Rat bone marrow stem cells isolation and culture as a bone formative experimental system. Bosn J Basic Med Sci 13(1):27–30. https://doi.org/10.17305/bjbms.2013.2409
Hisamatsu Y, Suzuki N, Masum AA, Shibuya A, Abe R, Sato A, Tanuma SI, Aoki S (2017) Cationic amphiphilic tris-cyclometalated iridium(iii) complexes induce cancer cell death via interaction with Ca2+-calmodulin complex. Generic. https://doi.org/10.1021/acs.bioconjchem.6b00627
Xiang H, Chen H, Tham HP, Phua SZF, Liu J-G, Zhao Y (2017) Cyclometalated iridium(III)-complex-based micelles for glutathione-responsive targeted chemotherapy and photodynamic therapy. ACS Appl Mater Interfaces 9(33):27553–27562. https://doi.org/10.1021/acsami.7b09506
Gupta A, Mandal D, Ahmadibeni Y, Parang K, Bothun G (2011) Hydrophobicity drives the cellular uptake of short cationic peptide ligands. Eur Biophys J 40(6):727–736. https://doi.org/10.1007/s00249-011-0685-4
Sakagami K, Masuda T, Kawano K, Futaki S (2018) Importance of net hydrophobicity in the cellular uptake of all-hydrocarbon stapled peptides. Mol Pharm 15(3):1332–1340. https://doi.org/10.1021/acs.molpharmaceut.7b01130
Piper K, Boyde A, Jones SJ (1995) Volumes of chick and rat osteoclasts cultured on glass. Calcif Tissue Int 56(5):382–389. https://doi.org/10.1007/BF00301607
Tian N et al (2019) A nuclear permeable Ru(ii)-based photoactivated chemotherapeutic agent towards a series of cancer cells: in vitro and in vivo studies. Dalton Trans 48(19):6492–6500. https://doi.org/10.1039/C9DT00441F
Azar DF, Audi H, Farhat S, El Sibai M, Abi-Habib R, Khnayzer RS (2017) Phototoxicity of strained Ru(II) complexes: is it the metal complex or the dissociating ligand? Dalton Trans 46(35):11529–11532
Loftus LM et al (2016) New RuII complex for dual activity: photoinduced ligand release and 1O2 production. Chem Eur J 22(11):3704–3708. https://doi.org/10.1002/chem.201504800
Negri LB, Martins TJ, da Silva RS, Hamblin MR (2019) Photobiomodulation combined with photodynamic therapy using ruthenium phthalocyanine complexes in A375 melanoma cells: effects of nitric oxide generation and ATP production. J Photochem Photobiol B 198:111564. https://doi.org/10.1016/j.jphotobiol.2019.111564
El Khoury M et al (2020) Malva pseudolavatera leaf extract promotes ROS induction leading to apoptosis in acute myeloid leukemia cells in vitro. Cancers. https://doi.org/10.3390/cancers12020435
Lincoln R et al (2013) Exploitation of long-lived 3il excited states for metal-organic photodynamic therapy: verification in a metastatic melanoma model. J Am Chem Soc 135(45):17161–17175. https://doi.org/10.1021/ja408426z
McCain J et al (2019) Photophysical properties and photobiological activities of ruthenium(ii) complexes bearing π-expansive cyclometalating ligands with thienyl groups. Inorg Chem 58(16):10778–10790. https://doi.org/10.1021/acs.inorgchem.9b01044
Xu L, Zhong N-J, Xie Y-Y, Huang H-L, Jiang G-B, Liu Y-J (2014) Synthesis, characterization, in vitro cytotoxicity, and apoptosis-inducing properties of ruthenium(ii) complexes. PLoS ONE 9(5):e96082. https://doi.org/10.1371/journal.pone.0096082
Zhang P, Chen J, Liang Y (2010) DNA binding, cytotoxicity, and apoptotic-inducing activity of ruthenium(II) polypyridyl complex. Acta Biochim Biophys Sin 42(7):440–449. https://doi.org/10.1093/abbs/gmq040
Jiang G-B et al (2020a) New ruthenium polypyridyl complexes functionalized with fluorine atom or furan: synthesis, DNA-binding, cytotoxicity and antitumor mechanism studies. Spectrochim Acta A Mol Biomol Spectrosc 227:117534. https://doi.org/10.1016/j.saa.2019.117534
Li Y et al (2019) Polypyridyl ruthenium(II) complex-induced mitochondrial membrane potential dissipation activates DNA damage-mediated apoptosis to inhibit liver cancer. Eur J Med Chem 164:282–291. https://doi.org/10.1016/j.ejmech.2018.12.041
Jiang G-B et al (2020b) Development of four ruthenium polypyridyl complexes as antitumor agents: design, biological evaluation and mechanism investigation. J Inorg Biochem 208:111104. https://doi.org/10.1016/j.jinorgbio.2020.111104
D’Arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43(6):582–592. https://doi.org/10.1002/cbin.11137
Łomzik M, Mazuryk O, Rutkowska-Zbik D, Stochel G, Gros PC, Brindell M (2017) New ruthenium compounds bearing semicarbazone 2-formylopyridine moiety: Playing with auxiliary ligands for tuning the mechanism of biological activity. J Inorg Biochem 175:80–91. https://doi.org/10.1016/j.jinorgbio.2017.07.006
Mebratu Y, Tesfaigzi Y (2009) How ERK1/2 activation controls cell proliferation and cell death: is subcellular localization the answer? Cell Cycle 8(8):1168–1175. https://doi.org/10.4161/cc.8.8.8147
Snezhkina AV et al (2019) ROS generation and antioxidant defense systems in normal and malignant cells. Oxid Med Cell Longev. https://doi.org/10.1155/2019/6175804
Acknowledgements
This work was funded by the Lebanese American University and the Lebanese National Council for Scientific Research (Ref: 02-01-18).
Funding
This work was funded by the School Research and Development Council at the Lebanese American University and the Lebanese National Council for Scientific Research (Ref: 05-06-14).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fayad, C., Audi, H., Khnayzer, R.S. et al. The anti-cancer effect of series of strained photoactivatable Ru(II) polypyridyl complexes on non-small-cell lung cancer and triple negative breast cancer cells. J Biol Inorg Chem 26, 43–55 (2021). https://doi.org/10.1007/s00775-020-01835-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-020-01835-7