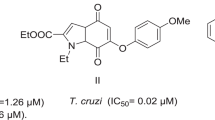
Abstract
An N-acylhydrazone scaffold has been used to develop new drugs with diverse biological activities, including trypanocidal activity against different strains of Trypanosoma cruzi. However, their mechanism of action is not clear, although in T. cruzi it has been suggested that the enzyme cruzain is involved. The aim in this work was to obtain new N-propionyl-N′-benzeneacylhydrazone derivatives as potential anti-T. cruzi agents and elucidate their potential mechanism of action by a molecular docking analysis and effects on the expression of the cruzain gene. Compounds 9 and 12 were the most active agents against epimastigotes and compound 5 showed better activity than benznidazole in T. cruzi blood trypomastigotes. Additionally, compounds 9 and 12 significantly increase the expression of the cruzain gene. In summary, the in silico and in vitro data presented herein suggest that compound 9 is a cruzain inhibitor.
Graphic abstract





Similar content being viewed by others
References
Rassi A, Rassi A, Marin-Neto JA (2010) Chagas disease. Lancet 375:1388–1402
Coura JR, Borges-Pereira J (2010) Chagas disease: 100 years after its discovery. A systemic review. Acta Trop 115:5–13
Schmunis GA, Yadon ZE (2010) Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop 115:14–21
Gascon J, Bern C, Pinazo MJ (2010) Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop 115:22–27
Briceno L, Mosca W (2016) Quello che non si cerca difficilmente si trova: La malattia di Chagas. G Ital Cardiol 17:343–347
Center for Disease Control and Prevention. Parasites-American Trypanosomiasis (also known as Chagas Disease). https://www.cdc.gov/parasites/chagas/. Accessed 20 Dec 2017
World Health Organization. Sustaining the drive to overcome the global impact of neglected tropical diseases: second WHO report on neglected diseases. https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis). Accessed 27 April 2020
Wilkinson SR, Taylor MC, Horn D, Kelly JM, Cheeseman I (2008) A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc Natl Acad Sci 105:5022–5027
Urbina JA (2010) Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop 115:55–68
Avila-Sorrosa A, Tapia-Alvarado JD, Nogueda-Torres B, Chacón-Vargas KF, Díaz-Cedillo F, Vargas-Díaz ME, Morales-Morales D (2019) Facile synthesis of a series of non-symmetric thioethers including a benzothiazole moiety and their use as effcient in vitro anti-trypanosoma cruzi agents. Molecules 24:3077
Avila-Sorrosa A, Bando-Vázquez AY, Alvarez-Alvarez V, Suarez-Contreras E, Nieto-Meneses R, Nogueda-Torres B, Vargas-Díaz ME, Díaz-Cedillo F, Reyes-Martínez R, Hernandez-Ortega S, Morales-Morales D (2020) Synthesis, characterization and preliminary in vitro trypanocidal activity of N-arylfluorinated hydroxylated-Schiff bases. J Mol Struct 1218:128520
Soeiro MNC, de Castro SL (2009) Trypanosoma cruzi targets for new chemotherapeutic approaches. Expert Opin Ther Targets 13:105–121
Doyle PS, Zhou YM, Hsieh I, Greenbaum DC, McKerrow JH, Engel JC (2011) The Trypanosoma cruzi protease cruzain mediates immune evasion. PLoS Pathog 7:1–11
Martinez-Mayorga K, Byler KG, Ramirez-Hernandez AI, Terrazas-Alvares DE (2015) Cruzain inhibitors: efforts made, current leads and a structural outlook of new hits. Drug Discov Today 20:890–898
Duarte CD, Barreiro EJ, Fraga CAM (2007) Privileged structures: a useful concept for the rational design of new lead drug candidates. Mini-Rev Med Chem 7:1108–1119
Welsch ME, Snyder SA, Stockwell BR (2010) Privileged scaffolds for library design and drug discovery. Curr Opin Chem Biol 14:347–361
Thota S, Rodríguez DA, de Pinheiro PSM, Lima LM, Fraga CAM, Barreiro EJ (2018) N-Acylhydrazones as drugs. Bioorg Med Chem Lett 28:2797–2806
Romeiro NC, Aguirre G, Hernández P, González M, Cerecetto H, Aldana I et al (2009) Synthesis, trypanocidal activity and docking studies of novel quinoxaline-N-acylhydrazones, designed as cruzain inhibitors candidates. Bioorg Med Chem 17:641–652
Massarico Serafim RA, Gonçalves JE, de Souza FP, de Melo Loureiro AP, Storpirtis S, Krogh R et al (2014) Design, synthesis and biological evaluation of hybrid bioisoster derivatives of N-acylhydrazone and furoxan groups with potential and selective anti-Trypanosoma cruzi activity. Eur J Med Chem 82:418–425
Elizondo-Jiménez S, Moreno-Herrera A, Reyes-Olivares R, Dorantes-González E, Nogueda-Torres B, Gamosa A, de Oliveira E et al (2017) Synthesis, biological evaluation and molecular docking of new benzenesulfonylhydrazone as potential anti-Trypanosoma cruzi agents. Med Chem 12:1–10
Norma Oficial Mexicana NOM-062-ZOO-1999 (1999) Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. In: Diario Oficial de la Federación
Brener Z (1962) Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo 4:389–439
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) Autodock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 16:2785–2791
Salentin S, Schreiber S, Haupt VJ, Adasme MF, Schroeder M (2015) PLIP: fully automated protein-ligand interaction profiler. Nucleic Acids Res 43:W443–W447
Mejía-Jaramillo AM, Fernández GJ, Palacio L, Triana-Chávez O (2011) Gene expression study using real-time PCR identifies an NTR gene as a major marker of resistance to benznidazole in Trypanosoma cruzi. Parasites Vectors 4:169
Araújo PR, Burle-Caldas GA, Silva-Pereira RA, Bartholomeu DC, daRocha WD, Teixeira SMR (2011) Development of a dual reporter system to identify regulatory cis-acting elements in untranslated regions of Trypanosoma cruzi mRNAs. Parasitol Int 60:161–169
Tavernelli LE, Motta MCM, Silva Gonçalves C, Santos da Silva M, Elias MC, Alonso VL et al (2019) Overexpression of Trypanosoma cruzi High Mobility Group B protein (TcHMGB) alters the nuclear structure, impairs cytokinesis and reduces the parasite infectivity. Sci Rep 9:1–16
Zhang L, Zheng XF, Linn G, Zhao K (2007) Synthesis of 1,3-Disubstituted N-Amino-1,2,3,4-tetrahydroisoquinolines. Synlett 3:0374–0380
Ok DJ, Yoon KS (2009) Cinchona-alkaloid-based organic catalyst and a process of preparing chiral arylamine by using the same. National Center for Biotechnology Information. PubChem Patent Summary for KR-20100128182-A. https://pubchem.ncbi.nlm.nih.gov/patent/KR-20100128182-A. Accessed 9 Oct 2020
Tian Z, Qingchun Z, Qi H (2019) The preparation method of parahydroxyben-zaldehyde benzoyl hydrazone. National Center for Biotechnology Information. PubChem Compound Summary for CID 136470051, 4-Hydroxybenzaldehyde benzoyl hydrazone. https://pubchem.ncbi.nlm.nih.gov/compound/4-Hydroxybenzaldehyde-benzoyl-hydrazone. Accessed 9 Oct 2020
Masahiro H, Tomohiro K, Akihiko M, Kenichi S, Shigeki K, Yoshihisa T (2005) Rubber and tires composition. National Center for Biotechnology Information. PubChem Compound Summary for CID 5539485, N-[(Z)-(4-Methoxyphenyl) methylideneamino]benzamide. https://pubchem.ncbi.nlm.nih.gov/compound/5539485. Accessed 9 Oct 2020
Luhua L, Ying L, Qian W, Pei L (2016) D-3-phosphoglycerate dehydrogenase allosteric inhibitor and use thereof. National Center for Biotechnology Information. PubChem Compound Summary for CID 5397591, N-[(Z)-(4-Fluorophenyl)methylideneamino]benzamide. https://pubchem.ncbi.nlm.nih.gov/compound/5397591. Accessed 9 Oct 2020
Ibrahim AS, Ismail W, Mohammed A A, Iqbal CM, Atia-Tul W, Saima R (2013) Heterocyclic schiff's bases as novel and new antiglycation agents. National Center for Biotechnology Information. PubChem Compound Summary for CID 5397599, N-[(Z)-(2-Nitrophenyl)methylideneamino]benzamide. https://pubchem.ncbi.nlm.nih.gov/compound/5397599. Accessed 9 Oct 2020
Machado-Silva A, Gonçalves Cerqueira P, Grazielle-Silva V, Ramos Gadelha F, de Figueiredo PE, Ribeiro Teixeira SM et al (2016) How Trypanosoma cruzi deals with oxidative stress: antioxidant defence and DNA repair pathways. Mutation Res Rev Mutation Res 767:8–22
Pontes Espíndola JW, de Oliveira Cardoso MV, de Oliveira Filho GB, Oliveira Silva DA, Magalhaes Moreira DR, Bastos TM et al (2015) Synthesis and structure activity relationship study of a new series of antiparasitic aryloxyl thiosemicarbazones inhibiting Trypanosoma cruzi cruzain. Eur J Med Chem 101:818–835
Sajid M, Robertson SA, Brinen LS, McKerrow JH (2011) Cruzain: the path from target validation to the clinic. Adv Exp Med Biol 712:100–115
Acknowledgements
This research was funded by Secretaria de Investigacion y Posgrado del Instituto Politecnico Nacional (SIP-20200491). Gildardo Rivera Sánchez and Benjamin Nogueda-Torres holds a scholarship from the “Comisión de Operación y Fomento de Actividades Académicas” (COFAA-IPN) and “Programa de Estímulos al Desempeño de los Investigadores” (EDI-IPN). José Carlos Espinoza-Hicks is thankful to the Consejo Nacional de Ciencia y Tecnología (CONACYT,Mexico) for a grant to purchase the NMR instrument (INFR-2014-01-226114).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Delgado-Maldonado, T., Nogueda-Torres, B., Espinoza-Hicks, J.C. et al. Synthesis and biological evaluation in vitro and in silico of N-propionyl-N′-benzeneacylhydrazone derivatives as cruzain inhibitors of Trypanosoma cruzi. Mol Divers 26, 39–50 (2022). https://doi.org/10.1007/s11030-020-10156-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10156-5