Abstract
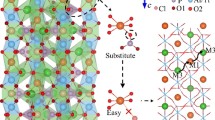
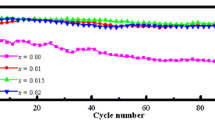
Attributed to the realization of the multielectron transfers, with a stable 3D framework, Li9V3 (P2O7)3(PO4)2 is considered an excellent cathode material, which can reversibly extract/insert nearly 5 mol Li. However, the poor intrinsic electronic conductivity and the low lithium diffusion coefficient limited its practical electrochemical ability. Considering the poor intrinsic electronic conductivity of the pristine Li9V3(P2O7)3(PO4)2 compound, the introduction of one extra electron by replacing V3+ with aliovalent Mo4+ ions increases the concentration of the electronic charge carriers from Li9V3(P2O7)3(PO4)2 and thus improves the electronic conductivity. At the same time, the larger ionic radius of Mo4+ leads to the increasement of the cell volume of the Li9V3(P2O7)3(PO4)2 sample and thus facilitates Li+ transport into the structure. As a result, the electrochemical performances of the Li9V3(P2O7)3(PO4)2 were improved obviously by a small amount of Mo. Especially for the Li9V3-xMox(P2O7)3(PO4)2 (x = 0.02) sample, the electrode present the highest specific capacity herein and an excellent rate performance which is a promising cathode for lithium ion battery application.









Similar content being viewed by others
References
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc 144(4):1188–1194
Okada S, Sawa S, Egashira M, Yamaki J, Tabuchi M, Kageyama H, Konishi T, Yoshino A (2001) Cathode properties of phospho-olivine LiMPO4 for lithium secondary batteries. J Power Sources 430:97–98
Liu C, Massé R, Nan X, Cho G (2016) A promising cathode for Li-ion batteries: Li3V2(PO4)3. Energy Storage Mterials 4:15–58
Ni Q, Zhang L, Bai Y, Liu T, Ren H, Xu H, Wu C, Lu J (2020) An extremely fast charging Li3V2(PO4)3 cathode at a 4.8 V cutoff voltage for li-Ion batteries. ACS Energy Lett 5:1763–1770
Tan L, Zhang S, Deng C (2015) Fast lithium intercalation chemistry of the hierarchically porous Li2FeP2O7/C composite prepared by an iron-reduction method. J Power Sources 275:6–13
Saidi MY, Barker J, Huang H, Sowyer JL, Adamson G (2003) Performance characteristics of lithium vanadium phosphate as a cathode material for lithium-ion batteries. J Power Sources 119-112:266–272
Saidi MY, Barker J, Huang H, Swoyer JL, Adamson G (2002) Electrochemical properties of lithium vanadium phosphate as a cathode material for lithium-ion batteries. Electrochem Solid State Lett 12:A149–A151
Huang H, Yin S, Kerr T, Taylor N, Nazar LF (2002) Nanostructured composites: a high capacity, fast rate Li3V2(PO4)3/carbon cathode for rechargeable lithium batteries. Adv Mater 21:1525–1528
Ni Q, Zheng L, Bai Y, Liu T, Ren H, Xu H, Wu C, Lu J (2020) An extreme fast-charging Li3V2(PO4)3 cathode at 4.8-V cut-off voltage for li-ion battery. ACS Energy Lett 5(6):1763–1770
Kim J, Yoo JK, Jung YS, Kang K (2013) Li3V2(PO4)3/conducting polymer as a high power 4 V-class lithium battery electrode. Adv Energy Mater 8:1004–1007
Harrison KL, Bridges CA, Segre CU, Varnado CD Jr, Applestone D, Bielawski CW, Paranthaman MP, Manthiram A (2014) Chemical and electrochemical lithiation of LiVOPO4 cathodes for lithium-ion batteries. Chem Mater 26(12):3849–3861
Kim S, Zhang Z, Wang S, Yang L, Cairns EJ, Penner-Hahn JE, Deb A (2016) Electrochemical and structural investigation of the mechanism of irreversibility in Li3V2(PO4)3 cathodes. J Phys Chem C 120(13):7005–7012
Kuang Q, Xu J, Zhao Y, Chen X, Chen L (2011) Layered monodiphosphate Li9V3(P2O7)3(PO4)2: a novel cathode material for lithium-ion batteries. Electrochim Acta 56(5):2201–2205
Balasubramanian P, Mancini M, Geßwein H, Geiger D, Axmann P, Kaiser U, Mehrens MW (2018) Kinetics and structural investigation of layered Li9V3(P2O7)3(PO4)2 as a cathode material for li-ion batteries. ChemElectroChem 5(1):201–210
Kuang Q, Zhao Y, Xu J (2011) Synthesis, structure, electronic, ionic, and magnetic properties of Li9V3(P2O7)3(PO4)2 cathode material for li-ion batteries. J Phys Chem C 115(16):8422–8429
Kaung Q, Lin Z, Zhao Y, Chen X, Chen L (2011) Lithium deintercalation behavior in Li-rich vanadium phosphate as a potential cathode for Li-ion batteries. J Mater Chem 21(38):14760–14765
Herle PS, Ellis B, Coombs N, Nazar LF (2004) Nano-network electronic conduction in iron and nickel olivine phosphates. Nat Mater 3(3):147–152
Xu J, Zhao Y, Kuang Q, Dong Y (2011) Preparation and electrochemical properties of Cr-doped Li9V3(P2O7)3(PO4)2 as cathode materials for lithium-ion batteries. Electrochim Acta 56(18):6562–6567
Lin X, Zhao Y, Kuang Q, Liang Z, Yan D, Liu X, Dong Y (2014) Synthesis and electrochemical properties of co-doped Li9V3(P2O7)3(PO4)2/C as cathode materials for lithium-ion batteries. Solid State Ionics 259:46–52
Zeng J, Zhao Y, Liang Z, Dong Y (2014) Synthesis and electrochemical properties of Li9V3−xTix(P2O7)3(PO4)2/C compounds via wet method for lithium-ion batteries. J Solid State Electrochem 18(2):561–567
Kuang Q, Zhao Y (2011) Synthesis and electrochemical properties of layered lithium monodiphosphate Li9V3−xAlx(P2O7)3(PO4)2 solid solutions. Electrochim Acta 58:296–302
Barker J, Gover R, Burns P, Bryan A (2007) The effect of Al substitution on the electrochemical insertion properties of the lithium vanadium phosphate, Li3V2(PO4)3. J Electrochem Soc 154:A307–A313
Xia Z, Zhang Y, Molokeev MS, Atuchin VV, Luo Y (2013) Linear structural evolution induced tunable photoluminescence in clinopyroxene solid-solution phosphors. Sci Rep 3:3310(1–7)
Funding
This work was supported by the Key-Area Research and Development Program of Guangdong Province (Grant No. 2019B090908002), Guangdong Natural Science Foundation of China (Grant Nos. 2017A030313269 and 2018A030313004), and the project of the Science and Technology Bureau from Dongguan Government (No. 2019622163008). The work was also supported by the Guangdong Provincial College Students Innovation and Entrepreneurship Training Program (Grant No. S202011845196).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duan, H., Wang, Z., He, B. et al. Improvement of electrochemical performance of the Li9V3(P2O7)3(PO4)2 cathode material by aliovalent Mo4+ doping. J Solid State Electrochem 25, 983–991 (2021). https://doi.org/10.1007/s10008-020-04873-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04873-y