Abstract
Wood packaging materials (WPMs) are widely used for collecting, storing and trading a wide range of products, including fresh fruit, vegetables and grains. The occurrence of moulds on WPMs used in the food industry must be avoided at every stage of the supply chain. This study aimed at (1) characterising fungal mould populations developing on fresh boards of hardwoods (European beech and poplar) and softwoods (Norway spruce and eastern white pine) commonly used by the packaging industry, and (2) assessing the effectiveness of two new molecules approved to come in contact with food, potassium sorbate and copper-8-quinolinolate, against mould growth and sporulation. A total of 322 fungal isolates belonging to 182 putatively different morphotypes were obtained. Spruce and beech boards were found to harbour a higher number of putatively different morphotypes compared to poplar and pine. The spectrum of fungi mostly included Ascomycota and the most abundant taxa were Trichoderma spp. and Penicillium spp. The effectiveness of the two new molecules (potassium sorbate approved for the use in both Europe and USA, and copper-8-quinolinolate approved for the use in USA only) was assessed on treated test pieces by inoculating conidial suspensions combining the three most common fungal species for each wooden material. Both preservatives showed comparable effectiveness and significantly reduced (P < 0.05) mould mycelial growth and sporulation on all the tested wooden materials compared to untreated controls, representing a suitable option for the control of moulds on WPMs.
Similar content being viewed by others
1 Introduction
Inefficient post-harvest procedures, including inadequate packaging for the wholesale market of fresh fruit, vegetables and grains, can cause severe economic losses to horticultural markets worldwide (Camargo and Perdas 2002; Cortez et al. 2002; Sharma et al. 2009). A range of materials can be used for fruit and vegetable packaging, including wooden products, corrugated cardboard and plastic. The most suitable packaging depends on the crop, the region, the length and nature of the market, the environmental conditions, the availability and costs of materials and the post-harvest procedures (Vigneault et al. 2006).
Crates, pallets and boxes are the major wood packaging materials (WPMs) for collecting, storing and trading a wide range of products, including fresh fruit, vegetables and grains. Eighty-five percent of goods are transported worldwide either on or within WPMs (FEFPEB Position Statement 2019). WPMs are manufactured of solid wood (e.g., sawn boards, blocks, etc.) or of engineered wood-based products (e.g., plywood, oriented strand board—OSB, medium-density fibreboard—MDF, etc.). Their success worldwide is related to the local availability, price, ease of processing and mechanical performance, considering that the use of wood enables to obtain light and robust structures suited to transport heavy loads (Andreolli et al. 2017). In addition, the environmental impact of WPMs is considerably lower compared to plastic packaging (Bergman et al. 2014; Kočí 2019). For the production of WPMs, a wide range of wood species can be used, including ash (Fraxinus spp.), beech (Fagus spp.), oak (Quercus spp.), and poplar (Populus spp.) among broadleaves, and pine (Pinus spp.) and spruce (Picea spp.) among conifers (Aviat et al. 2016).
Moulds are fungi growing on a large variety of substrates (Guynot et al. 2005) and mostly belonging to the genera Alternaria spp., Aspergillus spp., Cladosporium spp., Fusarium spp., Mucor spp., Penicillium spp., Rhizopus spp., and Trichoderma spp. (Samson 1985; Grant et al. 1989; Henz and Cardoso 2005; Clausen and Yang 2007; Yang and Clausen 2007). While the establishment of moulds commonly occurs by means of mitospores (i.e., conidia) which are ubiquitous in the air, optimal temperatures, high moisture conditions and nutrient availability may lead to the rapid growth of these fungi on wood and wood product surfaces. A number of papers have been published on this topic, with special emphasis on moulds growing on building materials (e.g., Nielsen 2003; Nielsen et al. 2004; Andersen et al. 2011; Johansson et al. 2012), including their adverse effects on human health (Dales et al. 1998; Koskinen et al. 1999; Purokivi et al. 2001). Recently, Lie et al. (2019) investigated how agar plate screening tests and water uptake tests can predict mould growth on exterior wooden claddings.
Since the 1990s, a number of scientific studies focused on wood in contact with food have been published to investigate the survival and the potential transfer from wood of microorganisms relevant to food hygiene and human health (Aviat et al. 2016 and references therein; Rico-Munoz 2017). Most of them targeted bacteria, while little is known about mould fungi.
Although the wood for hygienic reasons is in general deemed suitable for the use in the food industry, especially if compared with other packaging materials (Worfel et al. 1995; Schönwälder et al. 2000; Fink et al. 2013; Aviat et al. 2016), the occurrence of moulds on WPMs used in the food industry must be avoided at every stage of the supply chain (Sela et al. 2017; Snyder and Worobo 2018). Fungal growth, involving spore germination and hyphal extension eventually forming visible mycelium, makes the wood aesthetically and hygienically unacceptable to carry food products (Filip et al. 2012; Fink et al. 2013; Aviat et al. 2016 and references therein; Rico-Munoz 2017). First of all, moulds can modify organoleptic characteristics of fruit and vegetables and sometimes they are also responsible for post-harvest diseases (Kora et al. 2005). Furthermore, some mould genera such as Aspergillus, Penicillium and Fusarium produce metabolites (e.g., mycotoxins) associated with a range of human diseases (Nielsen 2003; Dao et al. 2008; Egbuta et al. 2017). Aspergillus niger Tiegh., Penicillium chrysogenum Thom., P. commune Thom. and P. expansum Link represent only some of the fungal species with potentially relevant implications for human health (Aviat et al. 2016 and references therein; Egbuta et al. 2017). Recently, the occurrence of spores of heat resistant moulds (e.g., Paecilomyces spp., Aspergillus spp., Talaromyces spp.) was demonstrated in food and beverage processing environments; in particular wooden pallets showed the highest spore counts (Rico-Munoz 2017).
Fungal growth is also responsible for the release of mitospores (i.e., conidia), thereby increasing the level of airborne inoculum and the dispersal potential of moulds. Indeed, mitospores of moulds are very tiny and light, which allow them to travel through the air (Carreras 2006; Yang et al. 2007a).
The quantification of microbial contamination of wooden surfaces can be performed by using different methods including agar-contact plate and swabbing (Miller 1996; Lortal et al. 2009) as well as stomacher, ultrasonic sound and brushing methods (Mariani et al. 2007; Le Bayon et al. 2010). The detection threshold of each of these methods depends on their recovery rate considering that wooden surfaces are porous and microorganisms can be trapped within cavities. However, no standard recovery methods are available for wooden surfaces and no scientific evidence shows that trapped microorganisms can be likely transferred to the surface again (Aviat et al. 2016).
In Europe, WPMs like other packaging are subjected to the European Regulation (EC) No (1935/2004) on materials and articles intended to come into contact with food, and to the Commission Regulation (EC) No (2023/2006) on good manufacturing practice for the above materials and articles. Based on these two regulations, contact materials must not transfer their constituents to food and must be manufactured according to the rules on Good Manufacturing Practices (GMPs) in order to preserve human health, to avoid unacceptable changes in the food, and to prevent the deterioration of its organoleptic characteristics. National measures have been adopted to cope with these general principles, providing detailed rules to fit the requirements for the specific uses of the different materials (e.g., DGCCRF 2012).
The International Standard for Phytosanitary Measures ISPM-15 (FAO 2017a) includes measures that are mandatory for importing WPMs into Europe and are intended to eradicate insects and quarantine microorganisms from wooden packaging. The commonly used heat treatment (HT) required by ISPM-15 hinges on exposing wood to a temperature of 56 °C for at least 30 min so that these parameters reach the central core of the material. While this treatment is generally effective in killing insects, it is not expected to prevent the growth of moulds, which rather may be favoured by the treatment conditions. HT process, in fact, draws moisture and sugar (food source) to the surface of the wood, which is also warm and wet: this determines ideal conditions for the growth of mould (Lambertz and Welling 2010; Iline et al. 2014; FAO 2017b). The kiln drying (KD) treatment used for drying wood at a moisture content lower than 15% is generally effective in containing fungal growth but it is rather expensive, making it an unsuitable method to avoid fungal contaminations.
An alternative approach to prevent mould growth on wood-based materials relies on treatments with non-toxic and non-volatile fungicides (Clausen and Yang 2007; Yang and Clausen 2007; Reinprecht 2010). An effective fungicide should prevent spore germination and increase the service life of wooden products under conditions of high humidity. Eco-friendly treatments based on the use of biological control agents or of natural extracts (e.g., plant extracts) represent intriguing alternatives to use of chemicals (Yang and Rossignol 1999; Yang et al. 2007a, b). However, when used on wood materials expected to come in contact with food, any treatment regardless of its origin must be sufficiently safe. In the last decades, experiments were performed to assess the effectiveness of different plant-associated substances such as flavonoids, tannins, and essential oils as safe preservatives against moulds, yeasts and bacteria (Rauha et al. 2000; Plumed-Ferrer et al. 2013). Laboratory tests were performed to assess the effectiveness of condensed tannins and potassium sorbate in reducing mycelial growth and sporulation of four fast-growing mould species [Penicillium chrysogenum, Trichoderma longibrachiatum Rifai, Cladosporium cladosporioides (Fresen) G. A. de Vries, and Aspergillus niger] developing on high-density fibreboard (HDF) used for the production of crates for fruit and vegetables (Giordano et al. 2011). The patterns of establishment and growth of moulds, in fact, can be considered similar in solid wood and wood-based products such as HDF.
In this study, fungal mould populations developing on boards obtained from the main hardwoods and softwoods used in the packaging industry were characterised. To the best of the authors’ knowledge, this is the first extensive investigation on mould associated with unseasoned boards (moisture content > 30%) for wooden packaging.
In addition, the effectiveness of two new molecules approved to come in contact with food was assessed in reducing mycelial growth and sporulation of the most common mould species on wooden packaging. This can be relevant to protect wooden packaging during storage after ISPM-15 heat treatment that, when performed on unseasoned timber, can lead to conditions favourable to the development of moulds. Experiments were performed in more extreme conditions, in terms of temperature and relative humidity, than that occurring in post-harvest to maximise moulds growth and sporulation.
2 Materials and methods
The overall experimental design is summarised in Table 1.
2.1 Wood material, fungal isolations and identification
The characterization of mould populations was conducted on fresh boards of 0.39 × 0.08 × 0.025 m (length, width, thickness) in size of four wood species commonly used by the packaging industry: two hardwoods, i.e. poplar (Populus spp.) and European beech (Fagus sylvatica L.), and two softwoods, i.e. eastern white pine (Pinus strobus L.) and Norway spruce [Picea abies (L.) Karst.]. Three to ten boards per each of the wood species were randomly sampled from eight wood-packaging manufacturers located in Northern Italy. The boards were directly taken from the manufacturers’ yards where they were stored in open-air for periods ranging from few days to some weeks, so that their moisture content was always > 30%. To avoid cross-contaminations, boards were singly placed in plastic bags, transferred to the laboratory and stored at 5 °C prior to testing.
Subsequently, the boards were individually sealed in a new plastic bag and incubated horizontally in the dark for 2 weeks in a growth chamber set at a temperature of 25 ± 1 °C. Sterilized filter papers dampened with sterile water were included in each bag to ensure high relative humidity throughout the incubation period. It should be emphasised that the above-mentioned experimental conditions were particularly favourable to mould growth and sporulation, and should be considered extremes with respect to ordinary conditions occurring during manufacture, storage and use of WPMs intended for food contact.
Fungal isolations were performed from boards by transferring small wood samples (0.005 × 0.005 × 0.002 m) onto 9 cm diameter Petri plates filled with 2% malt extract agar (MEA; 20 g malt extract, 20 g agar, 1000 ml distilled water). For each board, at least 20 wood samples were plated by taking them from areas where moulds were visible. To reduce fast-growing fungi without removing all superficial fungal inoculum, wood samples were previously surface disinfected by dipping in 5% sodium hypochlorite (NaClO) for 5 s and rinsed in sterile distilled water for 10 s. Petri plates were incubated in the light at room temperature (22 °C) for up to 2 weeks depending on the growth rates of the fungal colonies. Growing colonies were individually transferred to 2% MEA.
For each wood species, the purified fungal colonies were grouped in different morphotypes based on growth morphology and macroscopic features. Identification at the genus level was achieved by macro- and micro-morphological examination of colonies by using taxonomic guides and standard procedures (Pitt 1979; Domsch et al. 1980; von Arx 1981; Kiffer and Morelet 1997). The relative isolation frequency (IF) was computed as the percentage of colonies for each fungal morphotype on the total number of colonies obtained for each wood species.
Three major and representative fungal morphotypes were selected for each wood species to be used in the subsequent experiment on the effectiveness of treatments by combining the highest values of IF and the most distinctive morphological characters. All selected morphotypes were identified at species level based on macro- and micro-morphological features of colonies. To confirm the morphological identification, the fungal DNA was extracted and the internal transcribed spacer (ITS) region was amplified and sequenced with universal fungal primers ITS1 and ITS4 (White et al. 1990). Sequences were compared with those of known fungi using the National Center for Biotechnology Information’s GenBank nucleotide BLAST search.
2.2 Effectiveness of treatments
Test pieces of poplar, European beech, eastern white pine and Norway spruce (10 × 50 × 1 mm) were treated by dipping either in Celbrite FS2® (15% potassium sorbate as active ingredient; Koppers Performance Chemicals, Germany) for 20 min or in PQ-80® (10% solution of copper-8-quinolinolate as active ingredient; ISK Biocides Inc., Memphis, Tennessee) for 10 min. Currently, Celbrite FS2® is approved for the use in both Europe and USA, while PQ-80® in USA only. After dipping, test pieces were air-dried for 48 h. Treated and untreated test pieces (included in the experiment as controls) were sealed in plastic bags and sterilized with γ rays (2.5 Mrad) using Cobalt-60 radioisotopes at Gammatom (Guanzate–CO, Italy).
The effectiveness of treatments against moulds was assessed by inoculating treated and untreated test pieces with a mixed conidial suspension of the three selected fungal species for each wood species. Mixed conidial suspensions were primarily used to mimic “natural” contamination conditions during the service life of WPMs. In addition, mixed conidial suspensions were recommended to determine the spectrum of activity of new molecules or compounds for preservative/antimicrobial aims (Bush et al. 1946; Siegert 2012; Koziróg et al. 2016).
Subcultures of the above fungal species were prepared aseptically by transferring small pieces of mycelium or conidia masses from individual colonies to fresh 2% MEA. After incubation at room temperature for 10 days, conidia were collected in Eppendorf tubes (50 ml) after flooding the surface of the plates with 5 ml of sterile water. The concentration of conidia was assessed by using a counting Bürker chamber and each conidial suspension was standardized at 103 conidia/ml. The final suspension was prepared by mixing together the three conidial suspensions of three fungal species for each wood species.
According to Giordano et al. (2011), 10 test pieces treated with each of the two chemical preservatives and 10 untreated test pieces were singly and uniformly inoculated with 1 ml of each of the above conidial suspensions using a sterile Pasteur pipette. Ten untreated and non-inoculated test pieces were included in the experiment as controls to assess the level of natural fungal contamination.
After inoculations, test pieces were singly placed, horizontally, in 15 cm diameter Petri plates containing a piece of sterile filter paper dampened with 4 ml of sterile water, to prevent drying. The samples were incubated at room temperature for two weeks in the light. Samples were examined 7, 10 and 14 days after inoculations by visual estimation of the wood area colonized by the fungal mycelium, with a detection threshold corresponding to the graphics error (distance between two points), a value derived from the cartography field and conventionally equal to 0.2 mm. Given the crucial role played by airborne inoculum (i.e., conidia) in the post-harvest infection biology of these fungi, the effectiveness of treatments in reducing the sporulation ability of fungi was also assessed at the end of the experiment. The growing mycelium on each test piece was collected by rubbing with a sterile piece of gauze. The gauze was placed in a Falcon tube (50 ml) containing 10 ml of sterile water, and conidia were suspended by vortexing tubes for 1 min. Thanks to the distinctive morphological characteristics of three mixed fungal species, the concentration of conidia (conidia/ml) of each fungal species was determined by using a Bürker chamber as previously described; in this case, the detection threshold corresponds to the Bürker chamber detection limit (103 conidia/ml).
2.3 Data interpretation and analysis
The 95% bias corrected and accelerated confidence interval was calculated from 104 bootstrap samplings (DiCiccio and Efron 1996) to analyse the wood area colonized by the fungal mycelium and concentration of conidia, with the exception of those averages resulting from constant values (see results). Based on the above confidence intervals, treatment comparison was performed with the bootstrap hypothesis testing method described in Crawley (2013). Statistical analyses were conducted with R version 3.6.0 (R Core Team 2019).
3 Results and discussion
A total of 322 fungal isolates belonging to 182 putatively different morphotypes was obtained in this study (Online Resource 1). Ninety-nine additional isolates were contaminated, and it was not possible to obtain purified colonies for the identification. These isolates were excluded from all analyses.
Norway spruce and European beech boards were found to harbour a higher number of putatively different morphotypes, 74 and 68 corresponding to 155 and 92 fungal isolates, respectively, compared to boards of the other two wood species. An almost equal number of isolates (36) was obtained from poplar and eastern white pine, corresponding to 23 and 26 putatively different morphotypes, respectively (Online Resource 1).
One hundred fifty-six bacterial colonies were also counted of which 75 from Norway spruce, 50 from poplar, 16 from eastern white pine, and 15 from European beech. However, the above bacterial colonies were not identified since this study was not designed to investigate bacterial populations developing on boards.
The spectrum of fungi isolated from boards mostly included taxa belonging to Ascomycota; the only Zygomycota was Mucor sp., absent in eastern white pine boards but with IF > 8.0 in Norway spruce and poplar boards and IF > 1.0 in European beech boards. The most abundant taxon was Trichoderma spp. with 110 putatively different morphotypes followed by Penicillium spp. with 31 putatively different morphotypes. Trichoderma spp. and Penicillium spp., detected in both hardwoods and softwoods (Online Resource 1), included well-known fast-growing species previously reported to readily, and often preferentially, colonize solid wood materials under moisture conditions and in a temperature range conducive to spore germination (Flannigan and Miller 1993; Kang and Morrell 2000; Pasanen et al. 2000; Seifert and Frisvad 2000; Nielsen et al. 2004; Clausen and Yang 2007; Yang and Clausen 2007). It should be emphasised that two isolated fungal species, Penicillium commune and Aspergillus niger, are listed among those responsible for potentially relevant implications for human health (Aviat et al. 2016 and references therein; Egbuta et al. 2017).
This is the first extensive investigation on fungal mould populations associated with fresh-cut boards for wooden packaging. Previously, Henz and Cardoso (2005) assessed, under different RH conditions, the growth of fungi on the surface of solid pine wood for assembling the “K” box, a standard crate for packing, transporting and trading vegetables in Brazil. In their study, the predominant fungi growing on the wood surface were Trichoderma harzianum Rifai and Rhizopus stolonifer (Ehrenb.) Vuill. with small colonies of Aspergillus spp. and Penicillium spp.
All fungi showing high IF and selected to be used in the experiments to assess the effectiveness of treatments were identified at species level by combining morphological and molecular analyses (Table 2) and these were: Trichoderma longibrachiatum (IF: 14.0%), Mucor sp. (IF: 8.3%) and Trichothecium roseum (Pers.) Link (IF: 2.8%) for poplar; Trichoderma citrinoviride Bissett (IF: 5.4%), Aspergillus niger (IF: 7.6%) and Penicillium commune (IF: 3.3%) for European beech; Trichoderma harzianum (IF: 5.6%), Chaetomium globosum Kunze (IF: 16.7%) and Penicillium commune (IF: 2.8%) for eastern white pine; and Trichoderma atroviride P. Karst. (IF: 12.3%), Mucor sp. (IF: 8.4%) and Chaetomium globosum (IF: 2.6%) for Norway spruce. Previously, fungal genera such as Aspergillus, Penicillium and Trichoderma were used in other studies to test the effectiveness of chemical preservatives for the inhibition of moulds on wood in indoor applications (Price et al. 2002; Clausen and Yang 2003, 2005, 2007; Yang and Clausen 2007; Yang et al. 2007a; Tiitta et al. 2009).
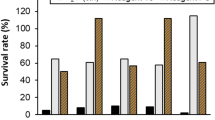
Mycelial growth and abundance of conidia on test pieces 14 days after inoculation are shown in Tables 3 and 4. It should be noted that mould growth remained undetected on untreated and non-inoculated test pieces. Celbrite FS2® and PQ-80® reduced significantly (P < 0.05) both the mycelial growth and the concentration of conidia of all the inoculated fungi compared to controls. The only exception was C. globosum whose conidia were not found on untreated test pieces of eastern white pine and Norway spruce. The reason of this exception is unclear since conidia were collected from actively growing colonies and because C. globosum was previously effectively used in mixed inoculation experiments with other fungal species, thereby excluding this species may be negatively affected by interspecific competition (Bush et al. 1946; Koziróg et al. 2016).
The present results are in agreement with Giordano et al. (2011) and Clausen and Yang (2003) who reported the effectiveness of potassium sorbate against fungal moulds on HDF samples used for fruit and vegetable crate production and on stakes of unseasoned southern pine, respectively.
Celbrite FS2® and PQ-80® were selected based on their previously reported antimicrobial and antifungal properties. Potassium sorbate, the active ingredient in Celbrite FS2®, is a common processed-food preservative with a broad-spectrum activity against moulds and yeasts (Sofos and Busta 1981; Al-Ashmawy and Ibrahim 2009). It is accepted worldwide for the use in food, such as cheese, wine, yogurt, dried meats, etc. (Marín et al. 2002). One of the main characteristics of this salt is that it doesn’t affect the taste, colour or flavour of food. PQ-80® is an effective and water-soluble fungicide to control sapstain and moulds on both hardwoods and softwoods. Although not approved for the use in European countries (Ruddick 2011), copper-8-quinolinolate, the active ingredient in PQ-80®, is the only U.S. Environmental Protection Agency (EPA)-registered preservative allowed by the U.S. Food and Drug Administration (FDA) for treatment of wood used in direct contact with fruit, vegetables and other foodstuffs, including pallets, boxes and bins, mushroom trays, and nursery trays and flats (Lebow 2010; CFR TITLE 21, § 178.3800 2019; EPA REG. NO. 1022-489-71581 2015).
Based on the present results, potassium sorbate and copper-8-quinolinolate were comparable in their effectiveness in reducing both the mycelial growth and the conidial production of moulds of WPMs. In both cases, no mould growth and fungal sporulation were observed. Their effectiveness was further supported by the fact that the present experimental conditions, in terms of temperature and relative humidity, were particularly favourable to mould growth and sporulation, and should be considered extremes with respect to ordinary conditions occurring in the post-harvest.
4 Conclusion
Although wood for hygienic reasons is in general deemed suitable for the use in the food industry, the compliance of WPMs for logistic operations related to food must be assured at each stage of the supply chain. In this context moulds can represent a serious threat not only to the integrity of fresh fruit, vegetables and grains, but also to the human health.
In the first case, moulds can be responsible for organoleptic modifications or post-harvest diseases; in the second one, some moulds (e.g., Penicillium spp., Aspergillus spp.) can have potentially relevant medical implications (e.g., mycotoxins).
In this study, a first extensive investigation on fungal mould populations associated with fresh boards of hardwoods and softwoods commonly used by the packaging industry was provided. The spectrum of fungi mainly included Ascomycota and the most abundant taxa were Trichoderma spp. and Penicillium spp. previously reported to readily, and often preferentially, colonize wood materials.
In addition, the effectiveness of two new molecules in reducing mycelial growth and sporulation of moulds was demonstrated, representing a suitable option for the control of moulds on WPMs. The tested molecules are potassium sorbate (Celbrite FS2®), available in both the EU and USA, and copper-8-quinolinolate (PQ-80®), currently available only in the USA, which is the producer’s reference market. Both products are safe in use and admitted for food contact.
To achieve a more complete picture of fungal mould populations associated with WPMs and to clarify their potential implications to the human health, further investigations are needed on different kinds of WPMs and during all manufacturing steps. Finally, from a practical perspective, the optimization of the application of potassium sorbate and copper-8-quinolinolate on industrial scale and the assessment of their persistence in the wooden packaging are also needed.
References
Al-Ashmawy MAM, Ibrahim JI (2009) Influence of potassium sorbate on the growth of yeasts and moulds in yogurt. Int J Dairy Technol 2:224–227
Andersen B, Frisvad JC, Søndergaard Ib, Rasmussen Ib S, Larsen LS (2011) Associations between fungal species and water-damaged building materials. App Environ Microb 77:4180–4188
Andreolli M, Corradetti D, Cremonini C, Negro F, Piazza M, Zanuttini R (2017) Italian standard UNI 9151—a new approach to the design of industrial packaging. Drvna Ind 68(3):267–273
Aviat F, Gerhards C, Rodriguez-Jerez JJ, Michel V, Le Bayon I, Ismail R, Federighi M (2016) Microbial safety of wood in contact with food: a review. Compr Rev Food Sci F 15:491–505
Bergman R, Puettman M, Taylor A, Skog K (2014) The carbon impacts of wood products. For Prod J 64(7/8):220–231
Bush V, Conant JB, Esselen GJ (1946) Tropical deterioration of equipment and materials. Summary technical report of the Tropical Deterioration Administrative Committee, NDRC, vol I. Washington DC
Camargo G, Perdas A (2002) Pós-colheita de verduras e frutas frescas (Post-harvest of fresh vegetables and fruits). In: Agriannual 2002. Anuário da Agricultura Brasileira, pp 41–42
Carreras E (2006) Preventing exposure to moulds. Clin Microbiol Infect 12(Supp. 17):77–83
CFR TITLE 21, § 178.3800 (2019) Code of Federal Regulations. Title 21, Volume 3. Chapter 1—food and drug administration, Department of Health and Human Services. Subchapter B – Food for human consumption (continued). Part 178—indirect food additives: adjuvants, production aids, and sanitizers. Subpart D – Certain adjuvants and production aids. Section 178.3800 Preservatives for wood. Revised as of April 1, 2019
Clausen CA, Yang VW (2003) Mold inhibition on unseasoned southern pine. International Research Group on Wood Preservation, Stockholm, Sweden IRG/WP/03-10465
Clausen CA, Yang VW (2005) Azole-based antimycotic agents inhibit mould on unseasoned pine. Int Biodeter Biodegrad 55:99–102
Clausen CA, Yang VW (2007) Protecting wood from mould, decay, and termites with multi-component biocide systems. Int Biodeter Biodegr 59:20–24
Commission Regulation (EC) No 2023/2006 of 22 December 2006 on good manufacturing practice for materials and articles intended to come into contact with food. Official J European Union L384/75 (2006)
Cortez LAB, Honório SL, Moretti CL (2002) Resfriamento de frutas e hortaliças (Cooling of fruits and vegetables). Embrapa Informação Tecnológica, Brasília
Crawley MJ (2013) The R book, 2nd edn. Wiley, Chichester
Dales R, Miller D, White J, Dulberg C, Lazarovits AI (1998) Influence of residential fungal contamination on peripheral blood lymphocyte populations in children. Arch Environ Health 53(3):190–195
Dao T, Bensoussan M, Gervais P, Dantigny P (2008) Inactivation of conidia of Penicillium chrysogenum, P. digitatum and P. italicum by ethanol solutions and vapours. Int J Food Microbiol 122:68–73
DGCCRF (2012) Note d’information n. 2012-93 Matériaux au contact des denrées alimentaires – cas du bois (Information note n. 2012-93 Materials in contact with foodstuffs - the wood case). In: Direction Générale de la Concurrence, de la Consommation et de la Répression des Fraudes. Ministère de l’Économie et des Finances, Paris
DiCiccio TJ, Efron B (1996) Bootstrap confidence intervals. Stat Sci 11:189–212
Domsch KH, Gams W, Anderson TH (1980) Compendium of soil fungi. Academic Press, London
Egbuta MA, Mwanza M, Babalola OO (2017) Health risks associated with exposure to filamentous fungi. Int J Environ Res Public Health 14(7):719
EPA REG. NO. 1022-489-71581. PQ-80. Product data sheet. Revised 7/9/2015
European Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC
FAO (2017a) International Standard for Phytosanitary Measures ISPM-15. Regulation of wood packaging material in international trade. Secretariat of the International Plant Protection Convention (IPPC Secretariat), Rome
FAO (2017b) Explanatory document for ISPM-15 (Regulation of wood packaging material in international trade). Secretariat of the International Plant Protection Convention (IPPC Secretariat), Rome
FEFPEB Position Statement (2019) “No deal” exit United Kingdom: statement for wooden pallets and packaging. FEFPEB Secretariat, 22 February 2019, Tilburg, The Netherlands
Filip S, Fink R, Oder M, Jevšnik M (2012) Hygienic acceptance of wood in food industry. Wood Sci Technol 46:657–665
Fink R, Filip S, Oder M, Jevšnik M (2013) Wood in food industry—potential applications and its limitations. In: Méndez-Vilas A (ed) Microbial pathogens and strategies for combating them: science, technology and education. Formatex, Badajoz, pp 188–194
Flannigan B, Miller JD (1993) Indoor humidity and the building envelope guidelines for evaluation of airborne microbial contamination of buildings. In: Rose WB, TenWolde A (eds) Bugs, mold and rott II. National Institute of Building Sciences, Washington, DC, pp 43–50
Giordano L, Nicolotti G, Zanuttini R, Cremonini C, Gonthier P (2011) Effectiveness of two chemical preservatives against widespread moulds of high density fibreboards. Eur J Wood Prod 69(4):667–669
Grant C, Hunter CA, Flannigan B, Bravery AF (1989) The moisture requirements of moulds isolated from domestic dwelling. Int Biodeterior 25:259–284
Guynot ME, Marìn S, Sanchis V, Ramos AJ (2005) An attempt to optimize potassium sorbate use to preserve low pH (4.5–5.5) intermediate moisture bakery products by modelling Eurotium spp., Aspergillus spp. and Penicillium corylophilum growth. Int J Food Microbiol 101:169–177
Henz GP, Cardoso FB (2005) Water absorption and fungi growth on pinewood used for crates for vegetable crops in Brazil. Hortic Bras 23:138–142
Iline II, Novoselov MA, Richards NK, Phillips CB (2014) Towards a test to verify that wood has been heat-treated to the ISPM-15 standard. N Z Plant Protect-Se 67:86–95
Johansson P, Ekstrand-Tobin A, Svensson T, Bok G (2012) Laboratory study to determine the critical moisture level for mould growth on building materials. Int Biodeter Biodegrad 73:23–32
Kang SM, Morrell JJ (2000) Fungal colonization of Douglas-fir sapwood lumber. Mycologia 92:609–615
Kiffer E, Morelet M (1997) Les deutéromycètes. Classification et clés d’identification générique (Deuteromycetes. Classification and genus identification keys). INRA Editions, Paris
Kočí V (2019) Comparisons of environmental impacts between wood and plastic transport pallets. Sci Total Environ 686:514–528
Kora C, McDonald MR, Boland GJ (2005) Occurrence of fungal pathogens of carrots on wooden boxes used for storage. Plant Pathol 54:665–670
Koskinen O, Husman T, Meklin T, Nevalainen A (1999) Adverse health effects in children associated with moisture and mould observations in houses. Int J Environ Heal R 9:143–156
Koziróg A, Rajkowska K, Otlewska A, Piotrowska M, Kunicka-Styczyńska A, Brycki B, Nowicka-Krawczyk P, Kościelniak M, Gutarowska B (2016) Protection of historical wood against microbial degradation—selection and application of microbiocides. Int J Mol Sci 17(8):1364
Lambertz G, Welling J (2010) Changes in extractives of Scots pine (Pinus sylvestris L.) after ISPM-15 heat treatment and their effect on fungal discolouration. Wood Mat Sci Eng 5(2):67–72
Le Bayon I, Callot H, Kutnik M, Denis C, Revol-Junelles AM, Millière JB, Giraud M, Gabillé M, Passédat N (2010) Development of microbiological test methods for the wooden packaging of foodstuffs. The Intl. Research Group on Wood Protection Publishing, Sweden
Lebow ST (2010) Wood preservation. In: U.S. Dept. of Agriculture, Forest Service (ed) Wood handbook: wood as an engineering material. General Technical Report FPL-GTR-190, pp 15.1-15.28
Lie SK, Vestøl GI, Høibø OA, Gobakken LR (2019) Surface mould growth on wood: a comparison of laboratory screening tests and outdoor performance. Eur J Wood Wood Prod 77:1137–1150
Lortal S, Di Blasi A, Madec M-N, Pediliggieri C, Tuminello L, Tanguy G, Fauquant J, Lecuona Y, Campo P, Carpino S, Licitra G (2009) Tina wooden vat biofilm: a safe and highly efficient lactic acid bacteria delivering system in PDO Ragusano cheese making. Intl J Food Microbiol 132(1):1–8
Mariani C, Briandet R, Chamba JF, Notz E, Carnet-Pantiez A, Eyoug RN, Oulahal N (2007) Biofilm ecology of wooden shelves used in ripening the French raw milk smear cheese Reblochon de Savoie. J Dairy Sci 90(4):1653–1661
Marín S, Guynot ME, Sanchis V, Arbonés J, Ramos AJ (2002) Aspergillus flavus, Aspergillus niger, and Penicillium corylophilum spoilage prevention of bakery products by means of weak-acid preservatives. J Food Sci Food Microbiol Saf 67:2271–2277
Miller A (1996) Wooden and polyethylene cutting boards: potential for the attachment and removal of bacteria from ground beef. J Food Protect 59(8):854–859
Nielsen KF (2003) Review: mycotoxins production by indoor moulds. Fungal Genet Biol 39:103–117
Nielsen KF, Holm G, Uttrup LP, Nielsen PA (2004) Mould growth on building materials under low water activities. Influence of humidity and temperature on fungal growth and secondary metabolism. Int Biodeter Biodeg 54(4):325–336
Pasanen AL, Kasanen JP, Sirpa R, Ikaheimo M, Rantamaki J, Kaariainen H, Kalliokoski P (2000) Fungal growth and survival in building materials under fluctuating moisture and temperature conditions. Int Biodeter Biodeg 46:117–127
Pitt JI (1979) The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press, London
Plumed-Ferrer C, Väkeväinen K, Komulainen H, Rautiainen M, Smeds A, Raitanen J-E, Eklund P, Willför S, Alakomi H-L, Saarela M, von Wright A (2013) The antimicrobial effects of wood-associated polyphenols on food pathogens and spoilage organisms. Int J Food Microbiol 164:99–107
Price D, Drago G, Noble J, Simmons R, Crow SJ, Ahearn D (2002) Rapid assessment of antimould efficacies of pressure-treated southern pine. J Ind Microbiol Biot 29:368–372
Purokivi MK, Hirvonen M-R, Randell JT, Roponen M, Meklin T, Nevalainen A, Husman T, Tukiainen HO (2001) Changes in pro-inflammatory cytokines in association with exposure to moisture-damaged building microbes. Eur J Epidemiol 18:951–958
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 15 Oct 2019
Rauha JP, Remes S, Heinonen M, Hopia A, Kähkönen M, Kujala T, Pihlaja K, Vuorela H, Vuorela P (2000) Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int J Food Microbiol 56(1):3–12
Reinprecht L (2010) Fungicides for wood protection—world viewpoint and evaluation/testing in Slovakia. Fungicides, Odile Carisse, IntechOpen. https://www.intechopen.com/books/fungicides/fungicides-for-wood-protection-world-viewpoint-and-evaluation-testing-in-slovakia. Accessed 14 January 2020
Rico-Munoz E (2017) Heat resistant molds in foods and beverages: recent advances on assessment and prevention. Curr Opin Food Sci 17:75–83
Ruddick JNR (2011) Use of chemicals to prevent degradation of wood. In: Winston Revie R (ed) Uhlig’s corrosion handbook, 3rd edn. Wiley, Hoboken, pp 469–480
Samson RA (1985) Occurrence of moulds in modern living and working environments. Eur J Epidemiol 1:54–61
Schönwälder A, Kehr R, Wulf A, Smalla K (2000) Wooden boards affecting the survival of bacteria? Holz Roh- Werkst 60:249–257
Seifert KA, Frisvad JC (2000) Penicillium on solid wood products. In: Samson RA, Pitt JI (eds) Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Hardwood Academic Publisher, Amsterdam, pp 285–298
Sela S, Schroeder T, Mamoru M, Ormsby M (2017) Explanatory document for ISPM-15 (Regulation of wood packaging material in international trade). Secretariat of the International Plant Protection Convention (IPPC), Rome
Sharma RR, Singh D, Singh R (2009) Biological control of postharvest diseases of fruit and vegetables by microbial antagonists: a review. Biol Control 50:205–221
Siegert W (2012) ISO 11930 - A comparison to other methods to evaluate the efficacy of antimicrobial preservation. SOFW-J 138:43–53
Snyder AB, Worobo RW (2018) Fungal spoilage in food processing. J Food Prot 81:1035–1040
Sofos JN, Busta FF (1981) Antimicrobial activity of sorbate. J Food Protect 44:614–617
Tiitta M, Tomppo L, Järnström H, Löija M, Laakso T, Harju A, Venäläinen M, Iitti H, Paajanen L, Saranpää P, Lappalainen R, Viitanen H (2009) Spectral and chemical analyses of mould development on Scots pine heartwood. Eur J Wood Prod 67:151–158
Vigneault C, Goyette B, Castro LR (2006) Maximum slat width for cooling efficiency of horticultural produce in wooden crates. Postharvest Biol Tec 40:308–313
von Arx JA (1981) The genera of fungi sporulating in pure culture. Cramer J, Vaduz
White TJ, Bruns TD, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Worfel RC, Sofos GC, Smith JB, Schmidt GR (1995) Microbial contamination of condensates formed on superstructure of wood and other materials in meat plants. Dairy Food Environ Sanit 15:430–434
Yang D-Q, Rossignol L (1999) Evaluation of Gliocladium roseum against wood-degrading fungi in vitro and on major Canadian wood species. Biocontrol Sci Technol 9:409–420
Yang VW, Clausen CA (2007) Antifungal effect of essential oils on southern yellow pine. Int Biodeter Biodegrad 59:302–306
Yang D-Q, Wan H, Wang X-M, Liu ZM (2007a) Use of fungal metabolites to protect wood-based panels against mould infection. Biocontrol 52:427–436
Yang D-Q, Wang X-M, Wan H (2007b) Biological protection of hardwood logs destined for panel manufacturing using Gliocladium roseum against biodegradation. Biocontrol 52:559–571
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement. This research was supported by FederlegnoArredo S.p.A. (Milano, Italy).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Giordano, L., Gonthier, P., Negro, F. et al. Effectiveness of new molecules against widespread moulds for food-safe hardwood and softwood packaging. Eur. J. Wood Prod. 79, 227–236 (2021). https://doi.org/10.1007/s00107-020-01626-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-020-01626-6