Abstract
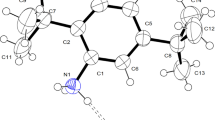
The synthesis, infrared, and crystal structure investigations of adamantane-1-ammonium picrate monohydrate, (C10H21N)(C6H2N3O7) ⋅ H2O, were performed. The title compound crystallizes in the space group P21/n with the monoclinic unit cell parameters: a = 12.7211(9) Å, b = 6.8233(4) Å, c = 21.4130(14) Å and β = 105.950(7)°, V = 1787.1(2) Å3, Z = 4. The asymmetric unit of the studied compound consists of one Adamantane-1-ammonium cation, one water and one picrate anion with two disordered nitro groups. The molecules are connected by strong H-bonds. As can be seen, the ionic pair is linked through two bifurcate N–H⋅⋅⋅O hydrogen bonds, identified as N1–H1A⋅⋅⋅O1 and N1–H1A⋅⋅⋅O2, to form a dimer. Also, water molecule acts bridging two picrate molecules connected by O1w–H1w⋅⋅⋅O1 and O1w–H2w⋅⋅⋅O4. These interactions form an infinite chain extended along the [101] direction. Finally, the main intermolecular contacts were analyzed based on Hirshfeld surfaces and their fingerprint plot.





Similar content being viewed by others
REFERENCES
N. A. Mir, R. Dubey, and G. R. Desiraju, IUCrJ 3, 96 (2016).
G. R. Desiraju, Angew. Chem. Int. Ed. 46, 8342 (2007).
K. Moradifard, Z. Derikvand, and A. Azadbakht, Crystallogr. Rep. 64, 1038 (2019).
J. C. Tenorio, R. S. Corrêa, A. A. Batista, and J. Ellena, J. Mol. Struct. 1048, 274 (2013).
R. S. Corrêa, F. T. Martins, J. Ellena, M. H. dos Santos, and A. C. Doriguetto, Acta Crystallogr. C 64, o395 (2008).
C. Ben Mleh, T. Roisnel, and H. Marouani, Crystallogr. Rep. 62, 246 (2017).
S. V. Tyutrina, M. A. Osina, N. V. Myasnikova, and F. M. Dolgushin, Crystallogr. Rep. 65, 247 (2020).
A. Vijayakumar, P. Arjun, A. Sinthiya, V. Duraipandiyan, and N. A. Al-Dhabi, Crystallogr. Rep. 62, 1035 (2017).
U. Likhitha, et al., J. Mol. Struct. 1211, 128052 (2020).
J. Pan, et al., RSC Adv. 5, 191 (2015).
J. F. Wyman, M. P. Serve, D. W. Hobson, L. H. Lee, and D. E. Uddin, J. Toxicol. Environ. Health 37, 313 (1992).
Y. Peng, A.-J. Zhang, M. Dong, and Y.-W. Wang, Chem. Commun. 47, 4505 (2011).
S. Nath, et al., New J. Chem. 42, 5382 (2018).
Goodman and Gilman’s the Pharmacological Basis of Therapeutics, Ed. by L. L. Brunton, J. S. Lazo, and K. L. Parker (McGraw Hill, 2006). https://doi.org/10.1036/0071422803
K. Smokrović and V. Stilinović, CrystEngComm 22, 1822 (2020).
G. M. Sheldrick, Acta Crystallogr. C 71, 3 (2015).
L. J. Farrugia, J. Appl. Crystallogr. 45, 849 (2012).
C. F. Macrae, et al., J. Appl. Crystallogr. 53, 226 (2020).
B. Ewald, Y. Prots, P. Menezes, and R. Kniep, Z. Krist. New Cryst. Struct. 219, 383 (2004).
I. M. Khan and S. Shakya, ACS Omega 4, 9983 (2019).
J. Zhang, et al. J. Mol. Struct. 1210, 127972 (2020).
D. N. Shetty, S. M. Kumar, and S. Abraham, Chem. Data Collect. 17–18, 442 (2018).
V. G. Saraswatula, D. Sharada, and B. K. Saha, Cryst. Growth Des. 18, 52 (2018).
R. S. Corrêa, M. H. dos Santos, T. J. Nagem, and J. Ellena, Struct. Chem. 21, 555 (2010).
R. S. Corrêa, M. H. dos Santos, T. J. Nagem, and J. Ellena, Struct. Chem. 23, 1809 (2012).
ACKNOWLEDGMENTS
FMN thanks PIVIC and PIP/UFOP for a fellowship. The authors thank the IFSC-USP (E.E. Castellano and J. Honorato) for X-ray crystallography measurements.
Funding
This work was supported by Brazilian agencies CNPq, Capes, and FAPEMIG. R.S.C. would like to thank the financial support provided by PROPP/UFOP, FAPEMIG (APQ-01674-18), and CNPq (grant nos. 403588/2016-2 and 308370/2017-1). Also, K.M.O. is supported by a postdoctoral fellowship grant from CAPES (PNPD program).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
All authors contributed to the study conception and design.
SUPPLEMENTARY MATERIAL
Crystallographic data been deposited with the Cambridge Crystallographic Data Centre as supplementary publication (CCDC 1997503). Copies of the data can be obtained, free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44-1223-336033; or e-mail: deposit@ccdc.cam.ac.uk).
Rights and permissions
About this article
Cite this article
Niquini, F.M., Moura, A.L., Machado, P.H. et al. Synthesis, Infrared and Molecular Structure of Adamantane-1-Ammonium Picrate Monohydrate: A Derivative of the Antiviral Symmetrel. Crystallogr. Rep. 65, 879–884 (2020). https://doi.org/10.1134/S1063774520060231
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774520060231