Abstract
We conducted several tests on the stingless bee Melipona quadrifasciata aiming to determine the impact of the glue used for applying radio frequency identification (RFID) tags on this bee species. The study was organized in three experimental sets, in which we evaluated the effects of a synthetic glue, a natural glue (shellac), and the effects of the bee manipulation alone (control group). We performed (i) an in vitro experiment (bioassay), in which we tested five different experimental treatments in triplicate: chip plus synthetic glue, chip plus shellac, synthetic glue, shellac, and control, totaling 150 bees (n = 30 per treatment); (ii) field experiments, in which we tested the RFID tracking system composed of RFID tags, reading units, antennas, and circuit boards; and (iii) the morphological and histochemical analyses of the flight muscles of bees collected from each experimental treatment (n = 5 per treatment) at 48 h after the beginning of bioassay. Use of the natural glue, as opposed to the synthetic glue, promoted an increase of the bees’ longevity while inhibiting detrimental impacts on their foraging activities, as observed by both the bioassay and field experiments. We found negative responses to the synthetic glue treatment combined with the electronic tags, showing that the natural glue induces less morphological damage to the flight muscles of tagged stingless bees.
Similar content being viewed by others
1 Introduction
The use of miniaturized tracking devices on bees has increased in the last decade in an attempt to better understand the dynamics, biology, and ecology of these important pollinators (Kissling et al. 2014; Nunes-Silva et al. 2018, and references therein). Radio frequency identification (RFID) tags present a good option for data acquisition, as they are low cost, are light enough to be carried by the bee, and many can be used simultaneously amongst a diverse cohort of bees as they can be identified individually (de Souza et al. 2018).
Although this technology has been used frequently on honeybees—Apis mellifera (e.g., Beyaert et al. 2012; Schneider et al. 2012; Tenczar et al. 2014; Bromenshenk et al. 2015; Susanto et al. 2018), little is known about the use of RFID on other bee species, even though there is some use with Brazilian stingless bees (e.g., de Souza et al. 2018; Nunes-Silva et al. 2019; Gomes et al. 2020), and even less is known regarding the effects of the application and manipulation of these devices on wild bees. It is not well understood the impact on the longevity of bees manipulated to affix the tag, the quality and type of glues utilized, and the additional stress caused by the constant presence of the tag.
Most of the surveys use cyanoacrylate synthetic glue to attach the electronic tags on the bees’ thorax without any documented issues to A. mellifera (Beyaert et al. 2012; Tenczar et al. 2014; Susanto et al. 2018) and Melipona fasciculata (Nunes-Silva et al. 2019). However, when we took preliminary tests with Melipona quadrifasciata (common name “mandaçaia”), we found that none of the tagged bees were able to come back to the colony after the release. Among the questions raised around the cause of this problem, we hypothesized about the potential harm of the synthetic glue used to attach the tags to bees. Schneider et al. (2012) used shellac to glue the electronic tags on A. mellifera, inspiring us to develop a non-toxic glue and test it with M. quadrifasciata, an important native Brazilian stingless bee endemic of the Atlantic Forest biome (Silveira et al. 2002).
It is important to better understand the potential and limitations of the use of RFIDs on bees, mainly on wild bee species, particularly at field experiments. We were interested in better understand effects of synthetic and natural glues on bees. To achieve this we analyzed several parameters on the stingless bee M. quadrifasciata and evaluated the viability of fitting RFID tags to this species.
2 Materials and methods
We conducted our study in three experiment sets. First, we performed an in vitro experiment (bioassay), in order to find if the tagging process would be responsible for decreasing the lifetime of bees and to identify to what extent this influence was caused by the application of RFID tags (chips) and/or by the glue used to apply them. The RFID tags (Hitachi Chemical Co. Ltd 2016) are 2.5 × 2.5 × 0.4 mm in size and 5.4 mg in weight and electronically registered with a series of unique hexadecimal numbers (de Souza et al. 2018). The tags are placed on the bee’s thorax, between the wings (Figure 1). Second, we performed field experiments, simulating as close as possible the real routine of a bee working in the hive. Therefore, we investigated if tagged bees would be capable of leaving and re-entering the hive, as well as the time spent away from the hive, and if the glue type used to apply the tags would influence this activity. Finally, we performed the morphological and histochemical analysis of the flight muscles, in order to find if the glue used to apply the tags would somehow affect the bees’ tissue.
2.1 In vitro experiment: survivorship and mortality analysis
The forager bees were captured from three different colonies from the meliponary of the Chácara Palmeiras (23° 31′ 33.38″ S, 47° 21′ 56.31″ W) in the beginning of the afternoon and transported to the laboratory. In the lab we divided the bees into plastic pots (250 mL) with small holes to allow the air to enter, each one containing a punctured plastic microtube (1.5 mL) with syrup (sugar solution: 50% water; 50% organic sugar). Bees were acclimatized until the next morning in an incubator (Eletrolab EL202/E) at 28 °C and 70% humidity. We tested five different experimental treatments in triplicate: chip plus synthetic glue (CG), chip plus shellac (CS), synthetic glue (Gl), shellac (Sh), and control (Ct), totaling 150 bees (n = 30 per treatment). We used three pots for each colony (triplicate) for each experimental treatment, each one of 15 pots containing 10 bees, so that each colony represents a replicate. An extra pot per experimental treatment (n = 10 bees/pot/treatment) was essayed together with the triplicate performed for a survival test, in order to collect bees for morphological and histochemical analysis of flight muscle (item III).
For the CG treatment we glued the RFID chip on the bees using cyanoacrylate synthetic glue (Super Bonder, Loctite—usually used for tag application—e.g., Beyaert et al. 2012; Tenczar et al. 2014; Susanto et al. 2018; Nunes-Silva et al. 2019). For the CS treatment we glued the tag on the bees using shellac composed of natural constituents made of 50% organic shellac and 50% grain alcohol (Figure 1a). For the Gl treatment we applied the synthetic glue on bee’s thorax. For the Sh treatment we applied the shellac on the bee’s thorax. For the Ct treatment we simulated the application of both the glue and the RFID chip. We calculated the average time of manipulation for each application, which was 53 s per bee, and took approximately the same time to simulate the manipulation in the Ct treatment. Before placing the glue and chip on the bee, each one was transferred to a small immobilization cage to minimize the stress that could have been generated by manual manipulation (Figure 1b). At the end of manipulation, each bee was individually kept in a tube (8.0 × 2.5 cm). After 1 h of resting inside the tube, the bee was transferred to the pot in order to compose the experimental unit (n = 10 bees per pot). This time was standardized in order to completely dry the glue or shellac on each bee before bringing them together, as they could try to remove the tags from each other or even attack themselves because of the strong smell of the glue. After all the bees had been transferred to the pots and kept inside the incubator, the bioassay began in order to determine the lifetime from each experimental treatment. During bioassay, syrup was offered ad libitum to bees and new syrup was provided daily. Every 24 h after the experiment set up, we checked the pots to count the number of dead individuals per pot. Thus, we did this monitoring daily, until the last bee was dead. The bees that died during the bioassay were removed from the pot.
2.2 Field experiment
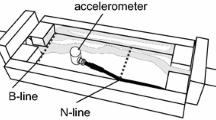
The field experiment was taken in a M. quadrifasciata hive at the UFSCar campus Sorocaba (47° 31′ 28″ W, 23° 34′ 53″ S). We tested the tracking system as described by de Souza et al. (2018), which was adapted to be applied in the M. quadrifasciata hive. The whole system is composed of RFID tags, reading units, antennas, and circuit boards. When the tagged bees pass by the antennas’ reach, the system records information on date, hour, and location within the hive entry of each individual bee (for more methodological details about the system, see de Souza et al. 2018). In order to best fit the M. quadrifasciata hive, the antennas were set as an extension of the entrance tube, simulated by a plastic tube (10 mm in diameter) protected inside a box (Figure 2). Two pairs of antennas were placed along the tube, one near to the entrance (pair A) of the hive and one near to the exit (pair B). Therefore, it is possible to identify if the bee is entering or exiting the hive according to the last registered position in the antennas.
Aiming to test the different glues plus chip under realistic conditions (in hive condition), in order to prove the data obtained in bioassay of survival (in vitro conditions), we divided the bees in plastic pots (250 mL) with small holes to allow the air entrance, each one containing a punctured microtube (1.5 mL) with sugar solution (50% water; 50% organic sugar). Bees were acclimatized until the next morning in an incubator (Eletrolab EL202/E) at 28 °C and 70% humidity; then the bees were released into the field. This experiment was performed twice, not simultaneously, being (I) one release of 50 bees glued with synthetic glue (Loctite) and (II) one release of 50 bees glued with shellac, made of 50% organic shellac and 50% grain alcohol. The tracking system in the hive registered the bees’ movement continuously, until there were no more entry data, which meant that all bees were dead.
2.3 Morphological and histochemical analysis
Bees were collected from each experimental treatment (n = 5 per treatment) at 48 h after the beginning of bioassay. These bees were anesthetized by cooling (4 °C) and dissected under a stereomicroscope at room temperature for the removal of flight muscles from the bee thorax, which were immersed in fixative solution (paraformaldehyde 4% in sodium phosphate buffer 0.1 M, pH 7.4) for 24 h at 4 °C.
After the fixation process, the organs were washed in a sodium phosphate buffer (0.1 M, pH 7.4) and submitted to slow dehydration in increasing ethanol solutions (4 °C), according to the methodology described by Silva-Zacarin et al. (2012). Five of the fixed and dehydrated organs were embedded in liquid historesin for a period of 24 h and then included in historesin with hardener to polymerize them. After, historesin blocks were submitted to microtomy to get histological sections of the organs (3-μm thickness) that was stained by hematoxylin and eosin for morphological analysis.
Histological sections of bee flight muscles were also submitted to the histochemical methods: Period-Acid-Schiff (PAS) for detection of neutral carbohydrate as glycogen (McManus 1946; Pearse 1960), bromophenol blue for protein detection (Pearse 1960), and Sudan black for lipid detection (Pearse 1960). Five slides were prepared for each individual containing 12 non-sequential histological sections per slide (n = 60 sections per individual, totaling 300 sections for each experimental treatment). In order to determine the qualitative morphological pattern of flight muscles, we analyzed 72 histological sections per treatment. For determining histochemical pattern according to each technique (PAS, Bromophenol blue, Sudan black), 36 non-sequential sections were analyzed per treatment.
2.4 Data analysis
To find if there were significant differences in the survival among the treatments in relation to the control for the in vitro experiment, we generated survivorship curves using the Cox proportional hazards regression model (survival package) in R studio (version 1.1.463). We performed lethal time (LT) analysis to estimate the time mortality of 10, 20, 30, 40, and 50% of the bees in each treatment using the R package ecotox. As we did not know the age of the tested flying bees, we decided not to include the analysis of the mortality over 50% in order to decrease the influences of mortality by aging.
Data about the field experiment was organized in Excel worksheets, and the number of bees detected by the RFID system per day was counted. We performed the Mann-Whitney test to check for significant differences in the mortality of the bees between the two different treatments of glue.
Histological and histochemical data were also analyzed semi-quantitatively in order to determine the morphological and histochemical patterns of thoracic flight muscles for each experimental treatment. Thus, three individuals were analyzed per experimental group. For each bee, 12 non-sequential histological sections per individual were randomly selected and analyzed, in order to determine the reaction pattern of organ in each experimental group and the histopathological alterations (lesions) to be scored, and their respective importance factor, according to Bernet et al. (1999) with adaptations for bees (Oliveira et al. 2019). Alterations (lesions) were classified into four levels of intensity, which were scored from 0 to 3, depending on the degree and/or frequency of them: 0—no alteration, 1—slight alteration, 2—moderate alteration, and 3—severe alteration. The importance factor was established for each lesion based on severity. This factor was categorized as (1) minimal pathological importance (repairable damage), (2) moderate pathological importance (damage was repairable in most cases), or (3) severe pathological importance (irreparable damage).
To determine histological and histochemical alterations in the bees’ muscles, two indices were calculated: the lesion index (Ile = level of intensity of lesion × pathological importance) and the organ index (Iorg = sum of the Ile indexes). Each individual was considered as a replicate from the experimental group (n = 3). The Wilcoxon/Kruskal-Wallis test was used to calculate the lesion index (Ile = level of intensity of lesion × pathological importance) due to the non-normal distribution of data as shown by the Shapiro. Afterwards, we did the Dunn test (post-test of Dunn's Kruskal-Wallis multiple comparisons with Bonferroni adjusted method) with p adjustment by the Bonferroni method. Because of the homogeneity (Barlett test) and normality of the data, we used Anova to analyze the total lesion index of the organ (Iorg = sum of the lesion indexes).
3 Results
3.1 In vitro experiment
The survivorship analysis (Figure 3) showed a very significant difference for the survival of the CG treatment in relation to the control (p < 0.001). The LT analysis highlighted the faster decay of bee lifetime in the CG treatment in relation to the other treatments, having a LT50 of only 65 h (2.7 days), while the control treatment presented a LT50 of 267 h (11 days) (Table I).
3.2 Field experiment
We observed that there was a significant difference between two glue treatments in the field experiment (p < 0.0001) in relationship to the number of bees that died. After the acclimatization period of the bees in the incubator, two bees tagged using shellac (natural glue) died, and one was found without the electronic tag. Therefore, 47 (94%) of acclimated bees were released in the field. After this procedure, on the first day of monitoring, 33 (70%) of released bees returned to the colony as detected by the RFID system. For this field treatment monitoring records occurred for 15 days.
Regarding the bees tagged using synthetic glue, 20 (40%) of them died during the acclimatization period inside the incubator and, therefore, 30 (60%) of the acclimated bees were released in the field. Of this total of bees released, only two (6.6%) were detected by the RFID system returning to the colony, and the records were obtained only during the first five days of field monitoring (Figure 4).
3.3 Morphological and histochemical analysis
3.3.1 Qualitative analysis
Flight muscles are composed of fibers of skeletal striated muscle that are multinucleated. In treatments exposed to synthetic glue (synthetic glue alone or associated with the chip) there were focal points of disorganization of the muscle fibers of the flight muscles and changes in the organization of sarcomeres (Figure 5). In treatments exposed to shellac glue (natural glue alone or associated with the chip), this disorganization was rarely observed and there was no sarcomere alteration (Figure 5).
Histological sections of flight muscle of Melipona quadrifasciata, stained with Hematoxylin and Eosin. a and b control. Normal morphological pattern showing intact muscle fibers. c and d shellac; e and f chip and shellac. Some focal degeneration of muscle fibers (fd) are visible with normal pattern of sarcomeres inside fibers. g and h synthetic glue; i and j chip and synthetic glue. Focal degeneration of muscle fibers (fd) are more frequent and some fibers have alterations in their sarcomeres (arrows).
In addition, bees tagged using synthetic glue presented glycogen accumulation in most of flight muscles fibers, contrary to the control samples that had no evidence of glycogen accumulation (Figure 6). Bees tagged using shellac presented glycogen sparsely distributed in muscle fibers (Figure 6). Bees exposed only to glue (synthetic glue or shellac) presented no glycogen accumulation (data not shown), similar to the control group. Regarding data obtained through the Bromophenol blue (protein detection) and Sudan Black (lipid detection) methods, there was no difference of positive labeling among treatments. The flight muscles presented a few droplets of lipids and great amount of proteins in their fibers.
Histological sections of flight muscles of Melipona quadrifasciata, submitted to Periodic-Acid-Schiff (PAS) reaction. a control. Muscle fibers are PAS-negative. b and c chip and synthetic glue. PAS-positive labeling is represented in magenta color; note the arrow in C indicating glycogen accumulation in muscle fiber. d and e chip and shellac. Some PAS-positive labeling is represented in magenta color in several muscle fibers.
3.3.2 Semi-quantitative analysis
The reaction pattern observed in the muscles was defined as a regressive change, according to Bernet et al. (1999), which involves degeneration of cellular structures of the tissue, including architectural and structural alterations (disorganization of muscle fibers; changes in the intracellular organization of sarcomeres). The intensity levels of alterations were defined according to the frequency of the lesion in muscle’s histological sections: score 0—less than 10% (< 10%), score 1—between 10 and 40% (≥ 10% and ≤ 40%), score 2—between 41 and 70% (> 40% and ≤ 70%), and score 3—higher than 70% (> 70%) of tissue. Separately, none of these lesion indexes (Ile) showed significative differences. However, the sum of the lesions per individual of each experimental group, which generated the organ total lesions index (Iorg), showed significant differences between the groups CG (chip + synthetic glue) and control (p = 0.0220177), and between the groups CG and Sh (shellac) (p = 0.0454423). In relation to histochemical data, we found significant differences in the stored glycogen between groups CG and control (p = 0.05012287), between CG and Gl (p = 0.05012287), and between CG and Sh (p = 0.05012287).
4 Discussion
In the in vitro experiment, bees from the control treatment lived up to 504 h (21 days) and took 267 h to reach a mortality of 50% of the individuals (LT50), while the CS, Gl, and Sh treatments took more than 240 h (10 days). Conversely, in the CG (chip and synthetic glue) treatment the LT50 was reached in 65 h (about 3 days). Our results show that despite the synthetic glue alone (Gl) having no significant effects in the in vitro experiments compared with the control, when the glue was combined with the application of RFID tags its negative effects were realized and caused the higher mortality of the bees. However, these side effects were not observed in either of the natural glue treatments—chip and shellac (CS) and shellac only (Sh).
Regarding the field experiments, the difference between the synthetic and the natural glue was undisputed, as bees from the CG treatment were registered for only five days, while bees from the CS treatment showed activity for 15 days. It is important to highlight that all individuals were sampled randomly during collection, without the knowledge of their actual age. Although we understand the occurrence of the physiological senescence of the flight muscles (Margotta et al. 2012), we aimed to simulate a field situation though the randomization of the flying bees. Even though, we had collected all bees with different ages, so that the randomization happened in all experimental groups, and we found statistical differences among the treatments. The average lifetime of an adult bee worker from the Melipona genus ranges from 40 to 52 days (Venturieri 2008), but lifetime is variable among the species; e.g., Melipona marginata is around 70 days, and average 30 days as foragers (Mateus et al. 2019). We found that the survival time of flying worker bees was similar to the colony condition for the genus (Mateus et al. 2019) when the natural glue (shellac) was used, showing that it is a better choice for adhering the electronic tags than the synthetic glue.
In the present study, we did not find signs of myofibril disruption in order to visibly distinguish sarcomeres in untreated, glue-treated, or chip-associated bees, although this characteristic may be present to a small extent in older forage workers, as observed by Correa-Fernandez and Cruz-Landim (2010) on the stingless bee Scaptotrigona postica. Therefore, myofibril alterations observed in M. quadrifasciata only in flying bees treated with synthetic glue, isolated or associated with the chip, showed negative effects because these bees presented muscle fiber disorganization in a greater proportion than that observed in bees treated with natural glue (isolated and associated with the chip). The negative effects of chip and synthetic glue were confirmed in both the survival analysis and LT50, under in vitro conditions, as well as in the field experiment where the difference in survival time of flying bees using synthetic glue was much smaller than those tested using shellac.
Under natural conditions, the decrease in the amount of stored glycogen in the muscle fibers and the appearance of stored lipid in these fibers distinguishes the nurse workers from the foragers, at least in Scaptotrigona postica and Apis mellifera (Cruz-Landim 2009), which reflects the transition from inside-nest to outside-nest tasks. In the present study, the flight muscles of untreated bees also did not have stored glycogen but had few droplets of lipids, as expected for flying bees. Instead, in tagged bees using natural glue (shellac) or synthetic glue, stored glycogen were visible in addition to lipid droplets. Interestingly, these glycogen were not present when glues were applied alone to the thorax of the bees. These results suggest an atypical glycogen storage in the tagged bees that could potentially modify their flight activities.
Moreover, associating this result with the survival data, bees tagged using synthetic glue had shorter survival time in vitro conditions, as well as a lower rate of return to the nest after its release in the field, that is, around 13% of the released live tagged bees. The combination of these two stressor agents (tag + synthetic glue) could potentially interfere in the glycogen oxidation, which plays a role in fueling flight (Suarez et al. 1996, Suarez et al. 2005), and/or in the restoration of muscle homeostasis damaged by the potentially toxic components of the synthetic glue. Future physiological and biochemical studies should better elucidate how these two stressors work together to cause the negative effects found. It is known that honeybee flight muscles undergo significant changes in biochemistry and gene expression and that these changes accompany a significant increase in the metabolic capacity during flight (Roberts and Elekonich 2005), but this information has not been provided until now for stingless bees. Biochemical and molecular studies should be performed in the future to explain this unexpected result and its implications for the flight of stingless bees M. quadrifasciata.
Glycogen is an easily mobilized energy source, and the flight muscles of bees oxidize this carbohydrate to supply their energy requirements during the transitions between rest and flight (Crabtree and Newsholme 1975; Sacktor 1976) or to extend flight range (Harrison 1986) in honeybees. In orchid bees (non-eusocial bee), glycogen may play a greater role in fueling flight in these species than in honeybees. Thus, despite the range of social behaviors displayed by bees, reliance upon carbohydrate oxidation by flight muscles is a feature common to all bee species that have already been studied (Neukirch 1982; Suarez et al. 2005). Pathways that provide energy for bee flight muscle include substrate oxidation, such as trehalose (carbohydrate) and proline (amino acid) released from fat body to hemolymph, which are absorbed by muscle fibers and participate in pathway of glycogen, as well as cytoplasmic and mitochondrial reactions inside muscle fibers (Suarez et al. 2005). In the present study, the data suggest that glycogen remained stocked in the muscle of flying bees with the use of synthetic glue, which results in its accumulation in muscle fibers, probably as a result of the absence or drastic decrease in flight muscles movement activity commonly present in forage bees.
It is concluded that natural glue (shellac) induces less damage to the flight muscles than synthetic glue (cyanoacrylate). By extension it is preferable to attach the electronic tags using the natural glue in order to reduce negative impact in longevity of stingless bees, allowing studies of native bees to be monitored over a larger time period.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Bernet, D., Schmidt, H., Meier, W., Burkhardt-Holm, P., Wahli, T. (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J. Fish Dis. 22(1), 25–34.
Beyaert, L., Greggers, U., Menzel, R. (2012) Honeybees consolidate navigation memory during sleep. J. Exp. Biol. 215(22), 3981–3988. doi: https://doi.org/10.1242/jeb.075499
Bromenshenk, J.J., Henderson, C.B., Seccomb, R.A., Welch, P.M., Debnam, S.E., Firth, D.R. (2015) Bees as biosensors: Chemosensory ability, honey bee monitoring systems, and emergent sensor technologies derived from the pollinator syndrome. Biosensors 5(4), 678–711. doi: https://doi.org/10.3390/bios5040678
Correa-Fernandez, F., Cruz-Landim, C. (2010) Differential Flight Muscle Development in Workers, Queens and Males of the Eusocial Bees, Apis mellifera and Scaptotrigona postica. J. Insect Sci. 10(1), 1-9. doi: https://doi.org/10.1673/031.010.8501
Crabtree, B., Newsholme, E.A. (1975) Comparative aspects of fuel utilization and metabolism by muscle. In: Insect Muscle (ed. P. N. R. Usherwood). Academic Press, London. pp. 405-491.
Cruz-Landim, C. (2009) Abelhas: morfologia e função de sistemas. UNESP, São Paulo. 408p. (ISBN 978-85-7139-927-3)
de Souza, P., Marendy, P., Barbosa, K., Budi, S., Hirsch, P., et al. (2018) Low-cost electronic tagging system for bee monitoring. Sensors 18(7), 1–21. doi: https://doi.org/10.3390/s18072124
Gomes, P.A.B., Suhara, Y., Nunes-Silva, P., Costa, L., Arruda, H., Venturieri, G., Imperatriz-Fonseca, V.L., Pentland, A., de Souza, P., Pessin, G. (2020) An Amazon stingless bee foraging activity predicted using recurrent artificial neural networks and attribute selection. Sci. Rep. 10, 9 doi: https://doi.org/10.1038/s41598-019-56352-8
Harrison, J.M. (1986) Caste-specific changes in honeybee flight capacity. Physiol. Zool. 59(2), 175-187. https://www.jstor.org/stable/30156031
Hitachi Chemical Co. Ltd. Product Specification for UHF Ultra Small Package Tag (IM5-PK2525), version 3.0; Hitachi Chemical Co. Ltd.: Tokyo, 2016.
Kissling, D.W., Pattemore, D.E., Hagen, M. (2014) Challenges and prospects in the telemetry of insects. Biol. Rev. 89(3), 511–530. doi: https://doi.org/10.1111/brv.12065
Margotta J.W., Mancinelli, G.E., Benito, A.A., Ammons, A., Roberts, S.P., Elekonich, M.M. (2012) Effects of Flight on Gene Expression and Aging in the Honey Bee Brain and Flight Muscle. Insects. 4(1), 9-30. doi: https://doi.org/10.3390/insects4010009.
Mateus, S., Ferreira-Caliman, M.J., Menezes, C., Grüter, C. (2019) Beyond temporal-polyethism: division of labor in the eusocial bee Melipona marginata. Insect. Soc. 66:317–328. doi: https://doi.org/10.1007/s00040-019-00691-2
McManus, J.F.A. (1946) Histological demonstration of mucin after periodic acid. Nature 158(202), 202-202. doi:https://doi.org/10.1038/158202a0
Neukirch, A. (1982) Dependence of the life span of the honeybee (Apis mellifica) upon flight performance and energy consumption. J. Comp. Physiol. 146, 35– 40.
Nunes-Silva, P., Hrncir, M., Guimarães, J.T.F., Arruda, H., Costa, L., Pessin, G., Siqueira, J.E., de Souza, P., Imperatriz-Fonseca, V.L. (2018) Applications of RFID technology on the study of bees. Insect. Soc. 66(1), 15-24. doi: https://doi.org/10.1007/s00040-018-0660-5
Nunes-Silva, P., Costa, L., Campbell, A.J., Contrera, F.A.L., Teixeira, J.S.G., et al. (2019) Radio Frequency Identification (RFID) reveals long distance flight and homing abilities of the stingless bee Melipona fasciculata. Apidologie. 51, 240–253 doi: https://doi.org/10.1007/s13592-019-00706-8
Oliveira, C.R., Domingues, C.E.C., de Melo, N.F.S., Roat, T.C., Malaspina, O., Jones-Costa, M., Silva-Zacarin, E.C.M., Fraceto, L.F. (2019) Nanopesticide based on botanical insecticide pyrethrum and its potential effects on honeybees. Chemosphere 236 (2019), 124282. https://doi.org/10.1016/j.chemosphere.2019.07.013
Pearse, A.G.E. (1960) Histochemistry Theoretical and Applied. Jet. Churchill Ltda., London. 530 p.
Roberts, S.P., Elekonich, M.M. (2005) Muscle biochemistry and the ontogeny of flight capacity during behavioral development in the honey bee, Apis mellifera. J. Exp. Biol. 208(22), 4193-4198. doi: https://doi.org/10.1242/jeb.01862
Sacktor, B. (1976) Biochemical adaptations for flight in the insect. Biochem. Soc. Symp. 41, 111-131.
Schneider, C.W., Tautz, J., Grünewald, B., Fuchs, S. (2012) RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera. PLoS One 7(1), 1–9. doi: https://doi.org/10.1371/journal.pone.0030023
Silva-Zacarin, E.C.M., Chauzat, M.P., Zeggane, S., Drajnudel, P., Schurr, F., Faucon, J.P., Malaspina, O., Engler, J.A. (2012) Protocol for optimization of histological, histochemical and immunohistochemical analyses of larval tissues: application in histopathology of honey bee. In: Méndez-Vilas, A. (Ed.). Current microscopy contributions to advances in science and technology. Badajoz: Formatex Research Center. p. 696-703.
Silveira, F.A., Melo, G.A.R., Almeida, E.A.B. (2002) Abelhas brasileiras: sistemática e identificação. Fundação Araucária, Belo Horizonte.
Suarez, R.K, Lighton, J.R.B., Joost, B., Roberts, S.P., Harrison, J.F. (1996) Physiology Energy metabolism, enzymatic flux capacities, and metabolic flux rates in flying honeybees. Proc. Natl. Acad. Sci. 93, 12616-12620.
Suarez, R.K., Darveau, C.A., Welch, K.C. Jr, O'Brien, D.M., Roubik, D.W., et al. (2005) Energy metabolism in orchid bee flight muscles: carbohydrate fuels all. J. Exp. Biol. 208: 3573-3579. doi: https://doi.org/10.1242/jeb.01775
Susanto, F., Gillard, T., de Souza, P., Vincent, B., Budi, S., et al. (2018) Addressing RFID Misreadings to Better Infer Bee Hive Activity. IEEE Access, 6, 31935–31949. doi: https://doi.org/10.1109/ACCESS.2018.2844181
Tenczar, P., Lutz, C., Rao, V., Goldenfeld, N., Robinson, G. (2014) Automated monitoring reveals extreme interindividual variation and plasticity in honeybee foraging activity levels. Anim. Behav. 95, 41–48. doi: https://doi.org/10.1016/j.anbehav.2014.06.006
Venturieri GC (2008) Criação de abelhas indígenas sem ferrão. Embrapa Amazônia Oriental, Belém. 60 p.
Acknowledgments
Authors thank the beekeeper Edson Sampaio for stingless bees’ supply and PhD. Leticia S. Souto from LADIVE (UFSCar Sorocaba) for the availability of the microtome (FAPESP 2015/01424-4). Authors thank Monique da Silva Souza for helping with the in vitro experiments and MSc. Rafaela Tadei for helping with the semi-quantitative analysis. Authors also thank MSc. Rafaela Tadei, PhD. Cristiane Ronchi de Oliveira, and PhD. Vanessa Bezerra de Menezes Oliveira for assisting with histological procedures.
Funding
Commonwealth Scientific and Industrial Research Organisation—CSIRO (RFID tags and reading system), Fundação de Amparo à Pesquisa do estado de São Paulo - FAPESP (process number 2017/21097-3), and Fundação para o Desenvolvimento Tecnológico da Engenharia—FDTE (process number 001505).
Author information
Authors and Affiliations
Contributions
RHT, MVNA, and ECMSZ conceived this research and designed experiments; RHT, MVNA, CIS, PM, PdS, and ECMSZ participated in the design and interpretation of the data; RHT organized and interpreted the field data; RHT and MVNA performed statistical analysis of the in vitro data; MVNA prepared the histological slides; ECMSZ performed the histological and histochemical analysis; All authors wrote the paper and participated in the revisions of it. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
The survey is in agreements with the ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Marina Meixner
Impact des colles utilisées pour les RFID sur la longévité et les muscles de vol de l'abeille sans dard Melipona quadrifasciata (Apidae : Meliponini)
système de suivi / analyse de survie / essais biologiques / marquage électronique / histopathologie
Der Einfluss von Leim für RFID-Markierungem auf die Lebensdauer und Flugmuskulatur der stachellosen Biene Melipona quadrifasciata (Apidae: Meliponini)
Tracking System / Lebensdaueranalyse / Biotest / elektronische Markierung /
Histopathologie
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toppa, R.H., Arena, M.V.N., da Silva, C.I. et al. Impact of glues used for RFIDs on the longevity and flight muscles of the stingless bee Melipona quadrifasciata (Apidae: Meliponini). Apidologie 52, 328–340 (2021). https://doi.org/10.1007/s13592-020-00823-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-020-00823-9