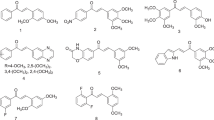
N,N'-bis[(1-aryl-3-heteroaryl)propylidene]hydrazine dihydrochlorides, P1, P4 – P8, and R1 – R7, were assayed against human oral squamous cell carcinoma (HSC-2, HSC-3, HSC-4), human promyelocytic leukemia cell line (HL-60), and human normal oral cells (HGF, HPC, and HPLF) as non-tumor cells to evaluate their cytotoxic properties. Peplomycin was used as a reference compound. It was found that P- and R-series hydrazone compounds exhibited cytotoxicity in a range of 11 ± 0.68 – 300 ± 1.0 ± M. Compound P1 which is a non-substituted hydrazone containing piperidine ring and compound R2 which is a 4-methyl hydrazone derivative containing pyrrolidine ring showed the most potent cytotoxic activity. These hydrazone compounds may serve as promising candidates for further studies.

Similar content being viewed by others
References
G. Stork and J. Benaim, J. Org. Synth., 6, 242 (1977).
A. C. Day and M. C. Whiting, J. Org. Synth., 6, 10 (1970).
E. Corey and D. Enders, Tetrahedron Lett., 17(1), 3 – 6 (1976).
E. Corey and D. Enders, Tetrahedron Lett., 17(1), 11 – 14 (1976).
S. Schöfer, J. Prak. Chem., 51, 185 (1850).
N. P. Belskaya, W. Dehaen, and V. A. Bakulev, ARKIVOC, Part (i), 275 – 332 (2010).
M. Asif and A. Husain, J. Appl. Chem., 2013, 1 – 7 (2013).
P. Vicini, M. Incerti, I. A. Doytchinova, et al., Eur. J. Med. Chem., 41(5), 624 – 632 (2006).
J. Mao, Y. Wang, B. Wan, et al., Chem. Med. Chem., 2(11), 1624 – 1630 (2007).
R. Narisetty, K. B. Chandrasekhar, S. Mohanty, et al., LDDD, 10(7), 620 – 624 (2013).
B. Evranos-Aksöz, S. Yabanoðlu-Çiftçi, G. Uçar, et al., Bioorg. Med. Chem. Lett., 24(15), 3278 – 3284 (2014).
V. Mashayekhi, K. H. M. E. Tehrani, S. Amidi, et al., Chem. Pharm. Bull., 61(2), 144 – 150 (2013).
Z. A. Kaplancýklý, L. Yurttaþ, A. Özdemir, et al., Med. Chem. Res., 23(2), 1067 – 1075 (2014).
E. B. Lindgren, M. A. de Brito, T. R. Vasconcelos, et al., Eur. J. Med. Chem., 86, 12 – 16 (2014).
K. Kucukoglu, M. Gul, M. Atalay, et al., Arzneimittelforschung, 61(6), 366 – 371 (2011).
K. Kucukoglu, H. I. Gul, R. Cetin-Atalay, et al., J. Enzyme Inhib. Med. Chem., 29(3), 420 – 426 (2014).
K. Kucukoglu, H. I. Gul, M. Gul, et al., LDDD, 13(8), 734 – 741 (2016).
K. Kucukoglu, M. Gul, H. I. Gul, et al., Med. Chem. Res., 27, 2116 – 2124 (2018).
M. Tramontini, Synthesis, 1973(12), 703 – 775 (1973).
M. Tramontini and L. Angiolini, Tetrahedron, 46(6), 1791 – 1837 (1990).
L. Racane, V. Traliæ-Kulenoviæ, Lelja Fiser-Jakic, et al., Heterocycles, 55(11), 2085 – 2098 (2001).
E. Kashiyama, I Hutchinson, and M. S. Chua, J. Med. Chem., 42(20), 4172 – 4184 (1999).
S. R. Bhusare, R. Pawar, and Y. B. Vibhute, Indian J. Het. Chem., 11(1), 79 – 80 (2001).
E. Mete, C. Ozelgul, C. Kazaz, et al., Arch. Pharm. (Weinheim), 343(5), 291 – 300 (2010).
E. Mete, H. I. Gul, S. Bilginer, et al., Molecules, 16(6), 4660 – 4671 (2011).
B. N. Singh, S. K. Shukla, and M. Singh, Asian J. Chem., 19(7), 5013 – 5018 (2007).
H. I. Gul, F. Sahin, M. Gul, et al., Arch. Pharm. (Weinheim), 338(7), 335 – 338 (2005).
M. Gul, M. Atalay, H. I. Gul, et al., Toxicol. In Vitro, 19(5), 573 – 580 (2005).
M. Ashok, B. S. Holla, and B. Poojary, Eur. J. Med. Chem., 42(8), 1095 – 1101 (2007).
H. I. Gul, H. Suleyman, and M. Gul, Pharmaceutical Biology, 47(10), 968 – 972 (2009).
Y. N. Şahin, B. Demircan, H. Süleyman, et al., Turk. J. Med. Sci., 40(5), 723 – 728 (2010).
M. Köksal, N. Gökhan, and E. Küpeli, et al., Arch. Pharm. Res., 30(4), 419 – 424 (2007).
D. Sriram, D. Banerjee, and P. Yogeeswari, J. Enzyme Inhib. Med. Chem., 24(1), 1 – 5 (2009).
M. Tugrak, H. I. Gul, K. Bandow, et al., Bioorg. Chem., 90, 103095 (2019).
H. I. Gul, M. Tugrak, M. Gul, et al., Bioorg. Chem., 90, 103057 (2019).
H. I. Gul, A. Demirtas, G. Ucar, et al., LDDD, 14(5), 573 – 580 (2017).
C. Yamali, H. I. Gul, H. Sakagami, et al., J. Enzyme Inhib. Med. Chem., 31(sup4), 125 – 131 (2016).
H. I. Gul, Z. Yazici, M. Tanc, et al., J. Enzyme Inhib. Med. Chem., 31(6), 1540 – 1544 (2016).
D. O. Ozgun, C. Yamali, H. I. Gul, et al., J. Enzyme Inhib. Med. Chem., 31(6), 1498 – 1501 (2016).
H. I. Gul, C. Yamali, A. T. Yasa, et al., J. Enzyme Inhib. Med. Chem., 31(6), 1375 – 1380 (2016).
S. Bilginer, H. I. Gul, E. Mete, et al., J. Enzyme Inhib. Med. Chem., 28(5), 974 – 980 (2013).
M. Tugrak, C. Yamali, H. Sakagami, et al., J. Enzyme Inhib. Med. Chem., 31(5), 818 – 823 (2016).
M. Tugrak, H. I. Gul, H. Sakagami, et al., LDDD, 12(10), 806 – 812 (2015).
K. O. Yerdelen, H. I. Gul, H. Sakagami, et al., J. Enzyme Inhib. Med. Chem., 30(3), 383 – 388 (2015).
K. O. Yerdelen, H. I. Gul, H. Sakagami, et al., LDDD, 12(8), 643 – 649 (2015).
H. I. Gul, M. Tugrak, and H. Sakagami, J. Enzyme Inhib. Med. Chem., 31(1), 147 – 151 (2016).
E. Unluer, H. I. Gul, A. Demirtas, et al., J. Enzyme Inhib. Med. Chem., 31(sup3), 105 – 109 (2016).
K. Kucukoglu, E. Mete, R. Cetin-Atalay, et al., J. Enzyme Inhib. Med. Chem., 30(4), 564 – 568 (2015).
C. Yamali, D. O. Ozgun, H. I. Gul, et al., Med. Chem. Res., 26(9), 2015 – 2023 (2017).
M. Tugrak, H. I. Gul, H. Sakagami, et al., Med. Chem. Res., 26(7), 1528 – 1534 (2017).
N. Motohashi, H. Wakabayashi, T. Kurihara, et al., Phytother. Res. 18(3), 212 – 223 (2004).
D. O. Ozgun, H. I. Gul, C. Yamali, et al., Bioorg. Chem., 84, 511 – 517 (2019).
M. Tugrak, H. I. Gul, H. Sakagami, et al., Bioorg. Chem., 81, 433 – 439 (2019).
H. I. Gul, C. Yamali, G. Gunesacar, et al., Med. Chem. Res., 27(10), 2366 – 2378 (2018).
H. I. Gul, C. Yamali, H. Sakagami, et al., Bioorg. Chem., 77, 411 – 419 (2018).
H. I. Gul, M. Tugrak, M. Gul, et al., Anticancer Agents Med. Chem., 18(12), 1770 – 1778 (2018).
C. Yamali, H. I. Gul, D. O. Ozgun, et al., Anticancer Agents Med. Chem., 17(10), 1426 – 1433 (2017).
H. I. Gul, E. Mete, S. E. Eren, et al., J. Enzyme Inhib. Med. Chem., 32(1), 169 – 175 (2017).
K. Kucukoglu, F. Oral, T. Aydin, et al., J. Enzyme Inhib. Med. Chem., 31(S4), 20 – 24 (2016).
H. I. Gul, M. Tugrak, H. Sakagami, et al., J. Enzyme Inhib. Med. Chem., 31(6), 1619 – 1624 (2016).
Acknowledgements
This study was supported by the Research Foundation of Ataturk University Erzurum (Turkey).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kucukoglu, K., Gul, H.I. & Sakagami, H. Evaluation of Cytotoxic Properties of N,N'-bis[(1-aryl-3-heteroaryl)propylidene]-hydrazine dihydrochlorides. Pharm Chem J 54, 784–787 (2020). https://doi.org/10.1007/s11094-020-02274-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02274-z