Abstract
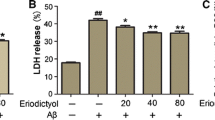
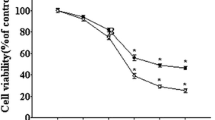
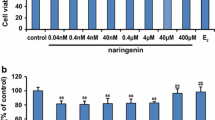
Abnormal excessive production and deposition of β-amyloid (Aβ) peptides in selectively susceptible brain regions are thought to be a key pathogenic mechanism underlying Alzheimer’s disease (AD), resulting in memory deficits and cognitive impairment. Genistein is a phytoestrogen with great promise for counteracting diverse Aβ-induced insults, including oxidative stress and mitochondrial dysfunction. However, the exact molecular mechanism or mechanisms underlying the neuroprotective effects of genistein against Aβ-induced insults are largely uncharacterized. To further elucidate the possible mechanism(s) underlying these protective effects, we investigated the neuroprotective effects of genistein against Aβ-induced oxidative stress mediated by orchestrating α7 nicotinic acetylcholine receptor (α7nAChR) signaling in rat primary hippocampal neurons. Genistein significantly increased cell viability, reduced the number of apoptotic cells, decreased accumulation of reactive oxygen species (ROS), decreased contents of malondialdehyde (MDA) and lactate dehydrogenase (LDH), upregulated BCL-2 expression, and suppressed Caspase-3 activity occurring after treatment with 25 μM Aβ25-35. Simultaneously, genistein markedly inhibited the decreases in α7nAChR mRNA and protein expression in cells treated with Aβ25-35. In addition, α7nAChR signaling was intimately involved in the genistein-mediated activation of phosphatidylinositol 3-kinase (PI3K)/Akt and Nrf2/keap1 signaling. Thus, α7nAChR activity together with the PI3K/Akt/Nrf2 signaling cascade likely orchestrates the molecular mechanism underlying the neuroprotective effects of genistein against Aβ-induced oxidative injury.







Similar content being viewed by others
References
Agrawal M, Saraf S, Saraf S, Antimisiaris SG, Chougule MB, Shoyele SA (2018a) Nose-to-brain drug delivery: an update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J Control Release 281:139–177
Agrawal M, Saraf S, Saraf S, Antimisiaris SG, Hamano N, Li SD et al (2018b) Recent advancements in the field of nanotechnology for the delivery of anti-Alzheimer drug in the brain region. Expert Opin Drug Deliv 15:589–617
Akaike A, Takada-Takatori Y, Kume T, Izumi Y (2010) Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of alpha4 and alpha7 receptors in neuroprotection. J Mol Neurosci 40:211–216
Alavi Naini SM, Soussi-Yanicostas N (2015) Tau hyperphosphorylation and oxidative stress, a critical vicious circle in neurodegenerative tauopathies? Oxid Med Cell Longev 2015:151979
Alexander A, Saraf S (2018) Nose-to-brain drug delivery approach: a key to easily accessing the brain for the treatment of Alzheimer’s disease. Neural Regen Res 13:2102–2104
Bagheri M, Roghani M, Joghataei MT, Mohseni S (2012) Genistein inhibits aggregation of exogenous amyloid-beta1-40 and alleviates astrogliosis in the hippocampus of rats. Brain Res 1429:145–154
Barik J, Wonnacott S (2006) Indirect modulation by alpha7 nicotinic acetylcholine receptors of noradrenaline release in rat hippocampal slices: interaction with glutamate and GABA systems and effect of nicotine withdrawal. Mol Pharmacol 69:618–628
Beal MF (2005) Oxidative damage as an early marker of Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 26:585–586
Biton B, Bergis OE, Galli F, Nedelec A, Lochead AW, Jegham S et al (2007) SSR180711, a novel selective α7 nicotinic receptor partial agonist: (1) binding and functional profile. Neuropsychopharmacol 32:1–16
Blass JP (2002) Alzheimer’s disease and Alzheimer’s dementia: distinct but overlapping entities. Neurobiol Aging 23:1077–1084
Butterfield DA (2002) Amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. Free Radic Res 36:1307–1313
Butterfield DA, Sultana R (2007) Redox proteomics identification of oxidatively modified brain proteins in Alzheimer’s disease and mild cognitive impairment: insights into the progression of this dementing disorder. J Alzheimers Dis 12:61–72
Caselli RJ, Knopman DS, Bu G (2020) An agnostic reevaluation of the amyloid cascade hypothesis of Alzheimer’s disease pathogenesis: the role of APP homeostasis. Alzheimers Dement. https://doi.org/10.1002/alz.12124
Chatterjee G, Roy D, Khemka VK, Chattopadhyay M, Chakrabarti S (2015) Genistein, the isoflavone in soybean, causes amyloid beta peptide accumulation in human neuroblastoma cell line: implications in Alzheimer’s disease. Aging Dis 6:456–465
Chen Z, Zhong C (2014) Oxidative stress in Alzheimer’s disease. Neurosci Bull 30:271–281
Chen X, Walker DG, Schmidt AM, Arancio O, Lue LF, Yan SD (2007) RAGE: a potential target for Abeta-mediated cellular perturbation in Alzheimer’s disease. Curr Mol Med 7:735–742
Cortes-Canteli M, Paul J, Norris EH, Bronstein R, Ahn HJ, Zamolodchikov D, Bhuvanendran S, Fenz KM, Strickland S (2010) Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer’s disease. Neuron 66:695–709
Dumont M, Ho DJ, Calingasan NY, Xu H, Gibson G, Beal MF (2009) Mitochondrial dihydrolipoyl succinyltransferase deficiency accelerates amyloid pathology and memory deficit in a transgenic mouse model of amyloid deposition. Free Radic Biol Med 47:1019–1027
Frozza RL, Horn AP, Hoppe JB, Simão F, Gerhardt D, Comiran RA et al (2009) A comparative study of beta-amyloid peptides Abeta1-42 and Abeta25-35 toxicity in organotypic hippocampal slice cultures. Neurochem Res 34:295–303
Glabe CG, Kayed R (2006) Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology 66:S74–S78
Gleason CE, Fischer BL, Dowling NM, Setchell KD, Atwood CS, Carlsson CM et al (2015) Cognitive effects of soy isoflavones in patients with Alzheimer’s disease. J Alzheimers Dis 47:1009–1019
Guo L, Duggan J, Cordeiro MF (2010) Alzheimer/’s disease and retinal neurodegeneration. Curr Alzheimer Res 7:3–14
Han Z, Li L, Wang L, Degos V, Maze M, Su H (2014) Alpha-7 nicotinic acetylcholine receptor agonist treatment reduces neuroinflammation, oxidative stress, and brain injury in mice with ischemic stroke and bone fracture. J Neurochem 131:498–508
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356
Henderson VW, St John JA, Hodis HN, Kono N, McCleary CA, Franke AA (2012) Long-term soy isoflavone supplementation and cognition in women: a randomized, controlled trial. Neurology 78:1841–1848
Hijioka M, Matsushita H, Ishibashi H, Hisatsune A, Isohama Y, Katsuki H (2012) a7 Nicotinic acetylcholine receptor agonist attenuates neuropathological changes associated with intracerebral hemorrhage in mice. Neuroscience 222:10–19
Ill-Raga G, Ramos-Fernández E, Guix FX, Tajes M, Bosch-Morató M, Palomer E et al (2012) Amyloid-β peptide fibrils induce nitro-oxidative stress in neuronal cells. J Alzheimers Dis 22:641–652
Jeong S (2017) Molecular and cellular basis of neurodegeneration in Alzheimer’s disease. Mol Cells 40(9):613–620
Jung KA, Kwak MK (2010) The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules 15:7266–7291
Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C et al (2016) Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: understanding the therapeutics strategies. Mol Neurobiol 53:648–661
Kang SW, Kim SJ, Kim MS (2017) Oxidative stress with tau hyperphosphorylation in memory impaired 1,2-diacetylbenzene-treated mice. Toxicol Lett 279:53–59
Kantarci K, Tosakulwong N, Lesnick TG, Zuk SM, Lowe VJ, Fields JA et al (2018) Brain structure and cognition 3 years after the end of an early menopausal hormone therapy trial. Neurology 90:e1404–e1412
Kem WR (2000) The brain alpha7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimer’s disease: studies with DMXBA (GTS-21). Behav Brain Res 113:169–181
Khurana R, Coleman C, Ionescu-Zanetti C, Carter SA, Krishna V, Grover RK, Roy R, Singh S (2005) Mechanism of thioflavin T binding to amyloid fibrils. J Struct Biol 151(3):229–238
Kim H, Xia H, Li L, Gewin J (2000) Attenuation of neurodegeneration-relevant modifications of brain proteins by dietary soy. BioFactors 12:243–250
LaFerla FM (2002) Calcium dyshomeostasis and intracellular signaling in Alzheimer’s disease. Nat Rev Neurosci 3:862–872
Li R, He P, Cui J, Staufenbiel M, Harada N, Shen Y (2013) Brain endogenous estrogen levels determine responses to estrogen replacement therapy via regulation of BACE1 and NEP in female Alzheimer’s transgenic mice. Mol Neurobiol 47:857–867
Liu LX, Chen WF, Xie JX, Wong MS (2008) Neuroprotective effects of genistein on dopaminergic neurons in the mice model of Parkinson’s disease. Neurosci Res 60:156–161
Lykhmus O, Voytenko L, Koval L, Mykhalskiy S, Kholin V, Peschana K et al (2015) Alpha7 nicotinic acetylcholine receptor-specific antibody induces inflammation and amyloid beta42 accumulation in the mouse brain to impair memory. PLoS ONE 10:e0122706
Matsuda S, Minami A, Ono Y (2015) Neuroprotection of genistein in Alzheimer’s disease. Academic Press, New York, pp 1003–1010
Naidu SD, Kostov RV, Dinkova-kostova AT (2014) Transcription factors Hsf1 and Nrf2 engage in crosstalk for cytoprotection. Trends Pharmacol Sci 36:6–14
Pardossi-Piquard R, Petit A, Kawarai T, Sunyach C, Alves da Costa C, Vincent B et al (2005) Presenilin-dependent transcriptional control of the Abeta-degrading enzyme neprilysin by intracellular domains of beta APP and APLP. Neuron 46:541–554
Picciotto MR, Zoli M (2008) Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. Front Biosci 13:492–504
Qian YS, Cao LG, Guan T, Chen L, Xin HB, Li YM et al (2015) Protection by genistein on cortical neurons against oxidative stress injury via inhibition of NF-kappaB, JNK and ERK signaling pathway. Pharm Biol 53:1124–1132
Reddy PH, Oliver DM (2019) Amyloid beta and phosphorylated tau-induced defective autophagy and mitophagy in Alzheimer’s disease. Cells 8:488
Ren BP, Liu YL, Zhang YX, Cai YQ, Gong X, Chang Y et al (2018) Genistein: a dual inhibitor of both amyloid β and human islet amylin peptides. ACS Chem Neurosci 9:1215–1224
Rickard DJ, Monroe DG, Ruesink TJ, Khosla S, Riggs BL, Spelsberg TC (2003) Phytoestrogen genistein acts as an estrogen agonist on human osteoblastic cells through estrogen receptors alpha and beta. J Cell Biochem 89:633–646
Schneider LS, Hernandez G, Zhao L, Franke AA, Chen YL, Pawluczyk S (2019) Safety and feasibility of estrogen receptor-β targeted phytoSERM formulation for menopausal symptoms: phase 1b/2a randomized clinical trial. Menopause 26:874–884
Song G, Ouyang G, Bao S (2005) The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med 9:59–71
Spagnuolo C, Moccia S, Russo GL (2018) Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur J Med Chem 153:105–115
Thal DR, Griffin WS, Braak H (2008) Parenchymal and vascular Abeta-deposition and its effects on the degeneration of neurons and cognition in Alzheimer’s disease. J Cell Mol Med 12:1848–1862
Tolar M, Abushakra S, Sabbagh M (2020) The path forward in Alzheimer’s disease therapeutics: reevaluating the amyloid cascade hypothesis. Alzheimers Dement. https://doi.org/10.1016/j.jalz.2019.09.075
Tsai TH (2005) Concurrent measurement of unbound genistein in the blood, brain and bile of anesthetized rats using microdialysis and its pharmacokinetic application. J Chromatogr 1073:317–322
Wu J, Liu Q, Tang P, Mikkelsen JD, Shen J, Whiteaker P et al (2016) Heteromeric α7β2 nicotinic acetylcholine receptors in the brain. Trends Pharmacol Sci 37:562–574
Yang H, Cong R, Luo N, Ju G, You SW (2010a) Long-term primary culture of highly-pure rat embryonic hippocampal neurons of low-density. Neurochem Res 35:1333–1342
Yang H, Cui GB, Jiao XY, Wang J, Ju G, You SW (2010b) Thymosin-beta4 attenuates ethanol-induced neurotoxicity in cultured cerebral cortical astrocytes by inhibiting apoptosis. Cell Mol Neurobiol 30:149–160
Yu JS, Cui W (2016) Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 143:3050–3060
Yu W, Mechawar N, Krantic S, Quirion R (2011) α7 Nicotinic receptor activation reduces β-amyloid-induced apoptosis by inhibiting caspase-independent death through phosphatidylinositol 3-kinase signaling. J Neurochem 119:848–858
Zhang X, Wu M, Lu F, Luo N, He ZP, Yang H (2014) Involvement of α7 nAChR signaling cascade in epigallocatechin gallate suppression of β-amyloid-induced apoptotic cortical neuronal insults. Mol Neurobiol 49:66–77
Zhu H, Itoh K, Yamamoto M, Zweier JL, Li YB (2005) Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett 579:3029–3036
Zhu H, Jia Z, Misra BR, Zhang L, Cao Z, Yamamoto M et al (2008) Nuclear factor E2-related factor 2-dependent myocardiac cytoprotection against oxidative and electrophilic stress. Cardiovasc Toxicol 8:71–85
Zoli M, Pucci S, Vilella A, Gotti C (2018) Neuronal and extraneuronal nicotinic acetylcholine receptors. Curr Neuropharmacol 16:338–349
Acknowledgements
The authors would like to acknowledge Jiaofei Zhang and Minghui Niu for excellent technical assistance. We would like to thank Dr. Sebastian Schmull for critical reading the manuscript and the English language review.
Funding
This work was funded by the National Natural Science Foundation of China (grants 81571208, 81772357, and 82071551), a key grant from the National Nature Science Foundation of China (81830077), Natural Science Foundation of Shaanxi Province (2020JM-686), and Xi’an Science and Technology Research Project (2019114913YX004SF037(3)).
Author information
Authors and Affiliations
Contributions
J.G. (Jianbin Guo), G.Y. (Guoqing Yang), and Y.H. (Yuqing He) involved in conceptualization; G.C. (Guihua Cao); Funding acquisition, D.H.(Dingjun Hao); Investigation, J.A. (Jing An), and R.Z. (Rui Zhang) participated in data curation,; M.X. (Huiming Xu) and H.F. (Hong Fan) contributed to methodology, ; L.Z. (Lingling Zhang) involved in collecting resources; and H.Y. (Hao Yang) did supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical Approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All experimental procedures in studies involving animals were performed in accordance with the ethical standards of the Animal Ethics Committee of Honghui Hospital of Xi’an Jiaotong University (No. 201712004).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, J., Yang, G., He, Y. et al. Involvement of α7nAChR in the Protective Effects of Genistein Against β-Amyloid-Induced Oxidative Stress in Neurons via a PI3K/Akt/Nrf2 Pathway-Related Mechanism. Cell Mol Neurobiol 41, 377–393 (2021). https://doi.org/10.1007/s10571-020-01009-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-020-01009-8