Abstract
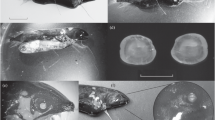
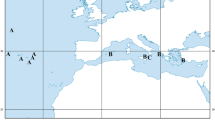
Deep-sea cephalopods are important in the bathyal ecosystems in terms of both abundance and diversity, but are seriously understudied. One of the most intriguing groups among the deep-sea cephalopods are Cirrata, relatively primitive octopods. Opisthoteuthis is the largest genus among the Cirrata. The least studied species of Opisthoteuthis in the Atlantic, Opisthoteuthis borealis Collins, 2005 was known from nine specimens only prior to our study, and nothing was described about its biology. Four males, all larger than the previously known maximum size (mantle length 78–96 mm cf. 75 mm), are described and COI sequence of the species provided to ease the identification of the Atlantic Opisthoteuthis. Our findings expand the known geographical (North Atlantic from 60° N northward and up to the Davis and Denmark Straits and the Iceland–Faroe Ridge), depth (878–1321 m) and temperature (3.0–3.6 °C) ranges of O. borealis. Arm bifurcation is reported in Cirrata for the first time, suggesting well-developed regeneration is present even in this ancient taxon of cephalopods. Ontogenetic increase of spermatophore length, i.e., when the spermatophores produced later during ontogenesis are larger than those produced earlier, is reported in Cirrata for the first time. The stomachs in all the studied specimens were at least one-third full, suggesting that O. borealis continues to feed and grow after reaching maturity. Polychaetes dominated over crustaceans in the stomach contents. Contrary to the assumption that Cirrata feed on relatively small prey only, large mature males of O. borealis consume polychaetes reaching 41.5–45.9% ML of the specimens.



Similar content being viewed by others
References
Abdullina LI, Gabidullina RI, Golikov AV, Sabirov RM (2015) Age structure of Lepidonotus squamatus and Harmothoe imbricata (Polychaeta, Polynoidae) population in sublittoral kelp ecosystems of Keret Archipelago. In: Golubkov SM (ed) Abstracts of 5-th International Scientific «Dynamics and functioning of aquatic ecosystems under the impact of climate change and antropogenic stress». Lema, St. Peterburg, pp 5–6
Aldred RG, Nixon M, Young JZ (1983) Cirrothauma murrayi Chun, a finned octopod. Philos T Roy Soc B 301:1–54
Boyle PR, Daly HI (2000) Fecundity and spawning in a deep-water cirromorph octopus. Mar Biol 137:317–324
Boyle PR, Collins MA, Williamson GR (1998) The cephalopod by-catch of deep-water trawling on the Hebrides slope. J Mar Biol Assoc UK 78:1023–1026
Breiby A, Jobling M (1985) Predatory role of the flying squid (Todarodes sagittatus) in north Norwegian waters. NW Atl Fish Organ Sci Coun Stud 9:125–132
Clarke MR (1962) The identification of cephalopod “beaks” and the relationship between beak size and total body weight. Bull Br Museum (Natural History) 8:419–480
Clarke MR (1996) Cephalopods as prey. III. Cetaceans. Philos T Roy Soc B 351:1053–1065
Collins MA (2003) The genus Grimpoteuthis (Octopoda: Grimpoteuthidae) in the north-east Atlantic, with descriptions of three new species. Zool J Linnean Soc 139:93–127
Collins MA (2005) Opisthoteuthis borealis: a new species of cirrate octopod from Greenland waters. J Mar Biol Assoc UK 85:1475–1479
Collins MA, Henriques C (2000) A revision of the family Stauroteuthidae (Octopoda: Cirrata) with redescriptions of Stauroteuthis syrtensis and S. gilchristi. J Mar Biol Assoc UK 80:685–697
Collins MA, Villanueva R (2006) Taxonomy, ecology and behaviour of the cirrate octopods. Oceanogr Mar Biol 44:277–322
Collins MA, O’Dea M, Henriques C (2001) A large Cirroteuthis magna (Cephalopoda: Cirrata) caught on the Cape Verde Terrace (North Atlantic). J Mar Biol Assoc UK 81:357–358
Collins MA, Laptikhovsky V, Strugnell JM (2010) Expanded description of Opisthoteuthis hardyi based on new specimens from the Patagonian slope. J Mar Biol Assoc UK 90:605–611
Cuccu D, Mereu M, Cannas R, Follesa MC, Cau A, Jereb P (2009) Morphology, biology and molecular characterizations of Opisthoteuthis calypso (Cephalopoda: Octopoda) from the Sardinian Channel (central western Mediterranean). J Mar Biol Assoc UK 89:1709–1715
Daly HI, Boyle PR, Collins MA (1998) Reproductive status of Opisthoteuthis sp. over an annual cycle. S Afr J Mar Sci 20:187–192
Ebersbach A (1915) Zur Anatomie von Cirroteuthis umbellata Fischer und Stauroteuthis sp. Zeitschrift. Z Wiss Zool 113:361–483
Fiorito G, Affuso A, Basil J, Cole A, de Girolamo P, D’Angelo L, Dickel L, Gestal C, Grasso F, Kuba M, Mark F, Melillo D, Osorio D, Perkins K, Ponte G, Shashar N, Smith D, Smith J, Andrews P (2015) Guidelines for the Care and Welfare of Cephalopods in Research–a consensus based on an initiative by CephRes, FELASA and the Boyd Group. Lab Anim 49(S2):1–90
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Golikov AV (2015) Distribution and reproductive biology of ten-armed cephalopods (Sepiolida, Teuthida) in the Barents Sea and adjacent areas. PhD dissertation, Moscow State University [in Russian]
Golikov AV, Morov AR, Sabirov RM, Lubin PA, Jørgensen LL (2013) Functional morphology of reproductive system of Rossia palpebrosa (Cephalopoda, Sepiolida) in Barents Sea. Proc Kazan Univ Nat Sci Ser 155:116–129 [in Russian with English abstract]
Golikov AV, Blicher ME, Jørgensen LL, Walkusz W, Zakharov DV, Zimina OL, Sabirov RM (2019) Reproductive biology and ecology of the boreoatlantic armhook squid Gonatus fabricii (Cephalopoda: Gonatidae). J Molluscan Stud 85:287–299
Grimpe G (1920) Teuthologische Mitteilungen V. Zwei neue Cirraten-Arten. Zool Anz 51:230–243
Guerra A, Villanueva R, Nesis KN, Bedoya J (1998) Redescription of the deep-sea cirrate octopod Cirroteuthis magna Hoyle 1885, and considerations on the genus Cirroteuthis (Mollusca: Cephalopoda). Bull Mar Sci 63:51–81
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Paleontol Electron 4:1–9
Healy JM (1993) Sperm and spermiogenesis in Opisthoteuthis persephone (Octopoda: Cirrata): ultrastructure, comparison with other cephalopods and evolutionary significance. J Molluscan Stud 59:105–115
Hochberg FG, Norman MD, Finn JK (2013) 2.2 Cirrate octopods. In: Jereb P, Roper CFE, Norman MD, Finn JK (eds) FAO Species Catalogue for Fishery Purposes, №4: Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Vol. 3. Octopods and Vampire Squids. FAO, Rome, pp. 244–267
Hoving HJT, Lipinski MR, Dam L (2010) The male reproductive strategy of a deep-sea squid: sperm allocation, continuous production, and long-term storage of spermatophores in Histioteuthis miranda. ICES J Mar Sci 67:1478–1486
Hoving HJT, Perez JAA, Bolstad KSR, Braid HE, Evans AB, Fuchs D, Judkins H, Kelly JT, Marian JEAR, Nakajima R, Piatkowski U, Reid A, Vecchione M, Xavier JCC (2014) The study of deep-sea cephalopods. Adv Mar Biol 67:235–359
Imperadore P, Fiorito G (2018) Cephalopod tissue regeneration: consolidating over a century of knowledge. Front Physiol 9:593
Jamieson AJ, Vecchione M (2020) First in situ observation of Cephalopoda at hadal depths (Octopoda: Opisthoteuthidae: Grimpoteuthis sp.). Mar Biol 167:82
Joubin L (1903) Sur quelques céphalopodes recueillis pendant les dernieres campagnes de S.A.S. le Prince de Monaco (1901–1902). Compte Rendus des Seances de l’Academic des Sciences 136:100–102
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Klages NTW (1996) Cephalopods as prey. II. Seals. Philos T Roy Soc B 351:1045–1052
Lindgren AR, Pankey MS, Hochberg FG, Oakley TH (2012) A multi-gene phylogeny of Cephalopoda supports convergent morphological evolution in association with multiple habitat shifts in the marine environment. BMC Evol Biol 12:129
Meyer WT (1906) Die Anatomie von Opisthoteuthis depressa (Ijima und Ikeda). Zeitschrift. Z Wiss Zool 85:183–269
Nigmatullin CM, Sabirov RM, Zalygalin VP (2003) Ontogenetic aspects of morphology, size, structure and production of spermatophores in ommastrephid squids: an overview. Berliner paläobiol Abh 3:225–240
Piertney SB, Huedelot C, Hochberg FG, Collins MA (2003) Phylogenetic relationships among cirrate octopods (Mollusca: Cephalopoda) resolved using mitochondrial 16S ribosomal DNA sequences. Mol Phylogenet Evol 27:348–353
Ramirez-Llodra E, Brandt A, Danovaro R, De Mol B, Escobar E, German CR, Levin LA, Martinez Arbizu P, Menot L, Buhl-Mortensen P, Narayanaswamy BE, Smith CR, Tittensor DP, Tyler PA, Vanreusel A, Vecchione M (2010) Deep, diverse and definitely different: unique attributes of the world’s largest ecosystem. Biogeosciences 7:2851–2899
Robison BH, Sherlock RE, Reisenbichler KR (2010) The bathypelagic community of Monterrey Canyon. Deep-Sea Res II 57:1551–1556
Rodhouse PG, Nigmatullin CM (1996) Role as consumers. Philos T Roy Soc B 351:1003–1022
Roper CFE, Voss GL (1983) Guidelines for taxonomic descriptions of cephalopod species. Mem Natl Museum Victoria 44:48–63
Sabirov RM (1995) Spermatophorogenesis and reproductive strategy in males of ommastrephid squids (Oegopsida: Ommastrephidae). PhD dissertation, Institute of Evolutionary Morphology [in Russian]
Sabirov RM (2010) Reproductive system in males of Cephalopoda. III. Spermatophores. Proc Kazan Univ Nat Sci Ser 152:8–21 [in Russian with English abstract]
Smale MJ (1996) Cephalopods as prey. IV. Fishes. Philos T Roy Soc B 351:1067–1081
Srivathsan A, Meier R (2012) On the inappropriate use of Kimura-2-parameter (K2P) divergences in the DNA-barcoding literature. Cladistics 28:190–194
Tanner AR, Fuchs D, Winkelmann IE, Gilbert MTP, Pankey MS, Ribeiro AM, Kocot KM, Halanych KM, Oakley TH, da Fonseca RR, Pisani D, Vinther J (2017) Molecular clocks indicate turnover and diversification of modern coleoid cephalopods during the Mesozoic Marine Revolution. P R Soc B 284:20162818
Toll RB, Binger LC (1991) Arm anomalies: cases of supernumerary development and bilateral agenesis of arm pairs in Octopoda (Mollusca, Cephalopoda). Zoomorphology 110:313–316
Vecchione M, Collins MA (2002) Systematics, ecology and biology of cirrate octopods: workshop report. Bull Mar Sci 71:79–96
Vecchione M, Mangold KM, Young RE (2016) (1922–2003). Cirrata Grimpe, 1916. Version 27 February 2016 (under construction). http://tolweb.org/Cirrata/20086/2016.02.27 in The Tree of Life Web Project (last accessed 13-Jul-2020)
Verrill AE (1883) Supplementary report on the “Blake” cephalopods. Bull Mus Comp Zool 11:105–115
Villanueva R (1992) Continuous spawning in the cirrate octopods Opisthoteuthis agassizii and O. vossi: features of sexual maturation defining a reproductive strategy in cephalopods. Mar Biol 114:265–275
Villanueva R, Guerra A (1991) Food and prey detection in two deep-sea cephalopods: Opisthoteuthis agassizii and O. vossi (Octopoda: Cirrata). Bull Mar Sci 49:288–299
Villanueva R, Collins MA, Sanchez P, Voss NA (2002) Systematics, distribution and biology of the cirrate octopods of the genus Opisthoteuthis (Mollusca, Cephalopoda) in the Atlantic Ocean, with description of two new species. Bull Mar Sci 71:933–985
Voss GL (1988) Evolution and phylogenetic relationships of deep-sea octopods (Cirrata and Incirrata). In: Clarke MR, Trueman ER (eds) The Mollusca, Volume 12, Paleontology and Neontology of Cephalopoda. Academic Press, San Diego, pp. 253–276
Voss GL, Pearcy WG (1990) Deep-water octopods (Mollusca: Cephalopoda) of the northeastern Pacific. Proc Calif Acad Sci 4th series 47:47–94
Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PD (2005) DNA barcoding Australia’s fish species. Philos T Roy Soc B 360:1847–1857
Young RE, Vecchione M, Donovan DT (1998) The evolution of coleoid cephalopods and their present biodiversity and ecology. S Afr J Mar Sci 20:393–420
Young RE, Vecchione M, Mangold KM (2001) (1922–2003). Cirrate male reproductive tract. http://tolweb.org/accessory/Cirrate_Male_Reproductive_Tract?acc_id=1486 in The Tree of Life Web Project (last accessed 13-Jul-2020)
Acknowledgements
We are grateful to ‘Initiating North Atlantic Benthos Monitoring (INAMon)’ project and BIOICE programme for providing parts of the samples, to the scientific groups and crews of the mentioned vessels, to T. P. Satoh (The Kyoto University Museum) for help with molecular works. INAMon was financially supported by the Greenland Institute of Natural Resources, North Atlantic Cooperation (nora.fo; J. nr. 510-151), Sustainable Fisheries Greenland, the Ministry for Research in Greenland (IKIIN) and the Environmental Protection Agency (Dancea) of the Ministry of Environment and Food of Denmark (J. nr. mst-112-00272). This research is also part of the Danish Presidency project in Nordic Council of Ministers, mapping seabed biodiversity and vulnerability in the Arctic and North Atlantic. We thank two anonymous reviewers of the manuscript and the editor Dr. Mike Vecchione for their valuable comments and suggestions to improve the quality of the paper.
Funding
This study is part of Initiating North Atlantic Benthos Monitoring, INAMon, which was financially supported by the Greenland Institute of Natural Resources, North Atlantic Cooperation (nora.fo; J. nr. 510-151), Sustainable Fisheries Greenland, the Ministry for Research in Greenland (IKIIN) and the Environmental Protection Agency (Dancea) of the Ministry of Environment and Food of Denmark (J. nr. mst-112-00272). This research is also part of the Danish Presidency project in Nordic Council of Ministers, mapping seabed biodiversity and vulnerability in the Arctic and North Atlantic. A.V.G. and G.G. acknowledge the financial support received from the BIOICE programme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for animal testing, animal care and use of animals were followed by the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgments, if applicable.
Data availability
All relevant data are included in the paper and/or in the supplementary information. Specimens are kept in the Greenland Institute of Natural Resources (PA-2017-7-68 and HM-2019-4-16) and the Icelandic Institute of Natural History (NI-21). A sequence of COI is openly accessible from GenBank (https://www.ncbi.nlm.nih.gov/genbank/) under accession number LC573902.
Author contributions
A.V.G. and R.M.S. designed the study; A.V.G., M.E.B., G.G., I.E.M. and D.V.Z. collected or provided the samples; A.V.G. and R.M.S. analysed the samples and respective data; J.Y.P. did molecular genetics part of the work; A.V.G., J.Y.P. and R.M.S. wrote the first draft of the paper; all authors were involved in interpreting the results and contributed to the final draft of the paper.
Additional information
Communicated by M. Vecchione
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 944 kb)
Rights and permissions
About this article
Cite this article
Golikov, A.V., Blicher, M.E., Gudmundsson, G. et al. Flapjack devilfish in the northern North Atlantic: morphology, biology and ecology of Opisthoteuthis borealis (Cephalopoda, Octopoda, Cirrata). Mar. Biodivers. 50, 108 (2020). https://doi.org/10.1007/s12526-020-01138-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-020-01138-9