Abstract
Resource and environmental safety protocols of incineration residues were evaluated by analyzing the metal concentration, heavy metal elution, desalination behavior, and chlorine removal ratio owing to particle size differences between bottom ash (BA) and grate sifting deposition ash (GA). In the total content test, Cl, Zn, and Cr in the incinerator BA exceeded the cement acceptance standard (Cl: 1000 mg/kg; Zn: 1700 mg/kg, and Cr: 170 mg/kg) at almost all of the particle sizes, while Au, Ag, Pd, and Zn had high contents in the GA. When using BA as a construction material, heavy metal elution values and contents are restricted as per the product quality standards based on the Japanese soil pollution control law. Lead within the BA and GA exceeded the standard values for most particle sizes. We predicted that there would be a limit on the elution of K by only washing with water. The removal ratio of total chlorine by particle size was approximately 20–70%, where the effect of the particle size on the removal ratio was small, suggesting that the elution of chlorine was complete in approximately 6 hours. These results contribute to information on the recycling of BA and GA.
Similar content being viewed by others
Introduction
In Japan, the final disposal amount of waste after intermediate treatment has decreased over time [1]. Whether the incineration residues are recycled or disposed of depends on the location of the intermediate treatment facilities, such as cement facilities and disposal sites [2]. Throughout Europe, the recycling of construction materials and metal resources has progressed in recent years by aging and weathering processes [3,4,5], as well as the physical sorting of municipal solid waste bottom ash [BA] [6,7,8,9].
When incineration residue is used as raw material for the process of preparing cement raw material with limestone, clay, and certain types of by-products or wastes, it is necessary to remove the salts [10], which prompts the use of desalination for fly ash and BA [11] because the leaching concentrations of Cl can induce reinforced steel corrosion in concrete material. However, BA experiences changes in its mineral form due to contact with water in the BA discharge system, which generates insoluble chlorine, known as Friedel’s salt (3CaO Al2O3 CaCl2 10H2O) [12,13,14,15], causing major reductions in the desalination efficiency. In addition, the construction of desalination equipment is expensive [2] and requires a large amount of water and wastewater treatment facilities. Thus, it is necessary to reduce the amount of desalination by separating only BA with high chlorine content via sieving and desalination.
In contrast, when using BA as a construction material, heavy metals, such as lead (Pb), are used as per the product quality standards, with reference to the elution test according to Environment Agency Notification No. 46 (JLT46) and the content testing standards according to the Ministry of the Environment Notification No. 19 (JLT19). Heavy metals are regarded as useful resources, not as repelled elements. In Europe, there have been significant improvements to the technologies used for concentrating and recovering metal resources by physical sorting from BA [7,8,9]. Through physical sorting, the recovery rate has increased by sieving based on the particle size, allowing for improved metal distribution and material flow [16, 17]. Grate sifting deposition ash (GA) [18], also known as grate siftings [19] or riddling ash [20], is a part of the BA that drops into gaps between the grates of the stoker type incinerator. GA containing a high concentration of useful metal resources is usually mixed with BA; however, in the U.S.A., GA is considered to be separate from BA [19]. In addition to the evaluation of metal resources according to particle size, it is necessary to evaluate the distribution of insoluble chlorine. However, the elemental content of sieved BA, desalination behavior, and evaluations of resources and environmental safety for GA are limited [18,19,20].
In this study, we focus on sieving, which is the most basic physical sorting technology. By separating and recovering only high-concentration salts and metal resources in the BA, the cost of pretreatment for cement raw material is reduced by reducing the amount of BA desalination, as well as yielding a reduction in the concentration of heavy metals, such as Pb. This will lead to the use of construction materials, recycling metal resources, and a reduction in the amount of the final disposal. This study also clarifies the behavior of metal concentration, elution, desalination, and insoluble chlorine depending on the particle size of the BA and GA, as well as a comprehensive evaluation of resource and environmental safety. As a result, the findings from this study can be used as a basis for examining the optimal resource recycling strategy according to the chemical characteristics of BA and GA.
Materials and methods
Sample analysis
Figure 1 shows two incinerator plants (plants A and B) and the sampling locations. Table 1 summarizes the outline of the survey plants and the analysis samples. The collected amount of each sample was approximately 5 kg. Combustible wastes pass through the drying zone, incinerating zone, and final incinerating zone from the inlet side and are quenched by water in the quench system while the melting furnace remains idle.
Schematic diagrams of the incinerators showing the six sampling locations: (a) plant A when the melting furnace is non-operational and (b) plant A when the melting furnace is operational. Plant A: (1) water-quenched mixed GA (QGA), (2) water-quenched BA (QBA), and (3) mixed BA through the dry-discharge system and the dried incineration BA once quenched (DDQBA). Plant B: (2) water-quenched BA (QBA), (4) unquenched drying zone GA (UDGA), (5) unquenched incinerating zone GA (UIGA), and (6) unquenched final incinerating zone GA (UFGA)
In plant A, when the melting furnace did not operate, the quenched BA was landfilled without drying. As the drying zone GA, incinerating zone GA, and final incinerating zone GA were unable to be collected owing to structural problems, water-quenched mixed GA (QGA) (1) was collected. The BA that contained the mixed GA was quenched (QBA) (2). When the melting furnace operated, BA through the dry-discharge system and BA once quenched through other incineration processes were mixed (DDQBA) (3) and collected. DDQBA was collected immediately before the melting furnace was shut down.
As plant B does not have a melting furnace, there is no dryer. Unquenched drying zone GA (UDGA) (4), unquenched incinerating zone GA (UIGA) (5), unquenched final incinerating zone GA (UFGA) (6), and quenched BA (QBA) (2) were collected. All samples were dried at 80 °C for 2 h, followed by the removal of iron scraps. Samples with all particle sizes and sieved samples (< 0.5, 0.5–2.0, 2.0–4.75, 4.75–9.5, and > 9.5 mm) were reduced with a condensation apparatus, a device for obtaining representative samples for analyses. Total content tests, 1 M hydrochloric acid extraction tests, heavy metal elution tests, and desalination tests were conducted. The 1M hydrochloric acid extraction, heavy metal elution, and desalination tests were conducted at plant A. A QBA value of 4.75 mm or more from plant A was not analyzed because there were insufficient samples.
Total content test
To evaluate repellent elements of the incineration residue for raw materials of cement, as well as a resource evaluation of the incineration residue for metal recovery, the sieved samples were finely pulverized by a high-speed vibration crusher (TI200, CMT), and sieved with an opening of 0.125 mm.
Main metal elements and nonmetal elements
Here, Fe, Al, Ca, K, S, P, Si, and Cl of the sieved samples were quantified using a Energy Dispersive X-ray Fluorescence System (XL3t-950S, RIGAKU). The quantification was carried out using the fundamental parameter method. The oxide converted value (excluding Cl) was compared with the acceptance standard for cement raw materialization [21]. All samples were quantified three times and the average value was calculated.
Trace metal element
Mercury was treated according to the nitric acid-sulfuric acid-potassium permanganate reflux decomposition method reported in the Japanese sediment survey method. A mixture of 10 mL of nitric acid, sulfuric acid (1 + 1), 50 g/L potassium permanganate solution, and 5 mL of potassium peroxodisulfate solution were added to 2.0 g of the crushed sample, heated for 2 h, and cooled. Then, 5 mL of a 100 g/L urea solution and 200 g/L hydroxyl ammonium chloride solution were added, and the solution was filtered using a glass fiber filter. The filtrate was separated and received 0.2 mL of sulfuric acid (1 + 1) and 0.2 mL of a 100 g/L tin (II) chloride solution. The sample was then introduced to a reduced vaporization atomic absorption device (RA-3, Japan Instruments Co., Ltd.). All elements (except Hg) were analyzed according to the temporary analysis method B of rare metals prepared by the Japan Society for Material Cycles and Waste Management [22]. Silver and the other elements were analyzed separately.
For Ag, a 1.0 g sample was moistened with water, the organic substance was decomposed with 1 mL of sulfuric acid and 5 mL of nitric acid and, after drying, 10 mL of 6 M hydrochloric acid was added to the sample before being dissolved through heating. After being filtered through 5B filter paper and washed with 6 M hydrochloric acid, the residue was discarded. The filtrate was adjusted to volume with 6 M hydrochloric acid, and an internal standard substance was added to prepare a test solution.
For elements other than Ag, 1.0 g of each sample was moistened with water; the organic substance was decomposed with 1 mL of sulfuric acid and 5 mL of nitric acid and, after drying, 10 mL of aqua regia was added to each sample before being dissolved through heating. After being filtered through 5B filter paper and washed with warm water, the filtrate was adjusted to volume and 10 mL of aqua regia and an internal standard substance were added to prepare a test solution. The residue was incinerated at approximately 550 °C, cooled, moistened with water, heated until dry using sulfuric and hydrofluoric acid, and then cooled again. The sample was further melted with sodium carbonate and boric acid, cooled, and dissolved by heating with nitric acid (1 + 1) to obtain a fixed volume and fractionated sample. Nitric acid (1 + 1) and an internal standard substance were added to prepare a test solution.
For Ag and other elements, the samples were collected twice at plant A and three times at plant B. These samples were analyzed once by via ICP mass spectrometry (7500 and 7700, Agilent Technologies), followed by the determination of their average values.
Elution test
To examine the possibility of using incineration residues as civil engineering materials, the following elution tests were conducted.
1 M hydrochloric acid extraction test
The incineration residue was analyzed in accordance with the soil content test (JLT19) reported in the Soil Contamination Countermeasures Law in Japan. The elements tested were Pb, Cd, and total Cr. A container holding 6 g of the sample and 200 mL of 1 M hydrochloric acid was shaken at a width of 4–5 cm approximately 200 times/min for 2 h. After standing for 10 min, the mixture was centrifuged at 3000 rpm for 20 min and the supernatant was filtered through a 0.45 μm diameter membrane filter to obtain a test solution. The amount of hydrochloric acid extraction was determined three times for each sample using an ICP emission spectrometer (Varian 720-ES, Agilent Technologies), followed by the determination of the average values.
Heavy metal elution test
In Japan, the elution test method for evaluating the safety of incineration residues and regenerated products is not defined (except for slags), but it is conventionally performed according to the soil content test (JLT46). This method ensures that soil lumps and aggregates are crushed and passed through a 2 mm sieve, such that it cannot be evaluated for each particle size. In addition, in the crushed sample, the elution amount increases more than that in a realistic environment, such that the original elution amount cannot be evaluated. For these reasons, it is desirable to make an assessment of its original state.
The elution tests were carried out in accordance with the JIS K 0058-1 chemical substance test method for slags-Part 1 elution amount test method 5 in its original state. A 50 g sample was weighed into a 1 L container made of PFTE, and 500 mL of purified water was added to produce a weight volume ratio of 10%. The mixture was stirred at a rotational speed of 200 times/min for 6 h. After centrifugation, the solution was filtered with a 0.45 μm membrane filter, and the filtrate was used as a test solution. The amount of elution was quantified for Pb, Cd, and Cr using an ICP emission spectrophotometer (Varian 720-ES, Agilent Technologies). The analysis was carried out three times, followed by the determination of the average values.
Desalination test
To confirm the desalination effect for each particle size, a desalination test by water elution was carried out for each sieved sample. The desalination test was performed by changing the stirring time to 6, 24, 48, 72, and 168 h for the entire sample with all particle sizes. Sodium, K, and Cl− in the eluate were quantified with an ion chromatograph (DX-500, Nippon Dionex Corporation).
The incineration residue before and after the demineralization test was pulverized by a high speed vibration crusher (TI200, CMT) and was passed through a 0.125 mm sieve. The total Cl was determined by the test for chloride ion content in hardened concrete (JIS A 1154) method, and the soluble Cl was quantified by the quantification of the warm water extracted chloride ion contained in the JIS A 1154 Annex B hardened concrete. The warm water temperature was 50 °C. The total Cl minus the soluble Cl was defined as insoluble chlorine. Each sample was analyzed twice, followed by the calculation of the average values. In addition, minerals, such as insoluble chlorine (Friedel’s salt), before and after desalination were identified from the peak at 2θ = 11.32°, with a radiation source Cu and a scan rate of 4º/min using a X-ray diffractometer (RINT-Ultima+, Rigaku).
Results and discussion
Particle size distribution
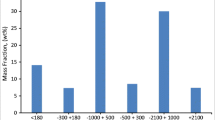
QBA showed similar particle size distributions at plants A and B, but GA tended to have a larger weight ratio for the larger particle size at plant B, as compared with plant A (Figs. 2 and 3). The 50% passage diameter (d50) of GA at plant A was approximately 2 mm, while that at plant B was approximately 5.5 mm. The difference in the particle size distribution of the GA is considered to be due to the size of the air jet or gap between the grate. In addition, the distribution at plant B between the GA collection zones was similar.
Total content test
The total content test data can be partially described as oxide according to the acceptance criteria for cement raw material production [21] (Table 2). Each cement plant has its own acceptance criteria, including for BA, but these data are not usually published. Several studies considering BA recycling have presented the acceptance criteria for BA. As the raw material composition of the cement plant depends on the amount of waste and by-products, other than the incineration residue and chemical composition, the acceptance criteria are not an absolute standard value, but are a guideline.
For the use of BA for cement raw materials, we first focused on QBA, which is a common sample from both plants A and B (Table 3). For Pb, Cu, and Zn, although the particle size range was slightly different, plant A showed almost the same content as the data from previous studies, while plant B showed the same or slightly higher content [23]. Chlorine almost exceeded the acceptance criteria (= 1000 mg/kg) for most particle sizes, where the content tended to be highest for the small particle size of ≤ 2.0 mm. There were also indications that the acceptance criteria for Cl are too strict [21]. For K2O, the difference in content due to the particle size was small and similar to the acceptance criteria, such that it may be greatly exceeded depending on variations in the samples. For SO3, the content at the small particle size of ≤ 2.0 mm in plant B for QBA is high and near the acceptance criteria, but it is significant below the acceptance criteria at the large particle size of > 2.0 mm. Both P2O5 and TiO2 were below the acceptance criteria for both particle sizes. Both Zn and Cr exceeded the acceptance criteria at most particle sizes, while Cd and Hg were below the acceptance criteria for all particle sizes.
Precious metals and main base metals were examined from the perspective of recovering useful metals in GA (Table 4), as compared with QBA (Table 3). Care must be taken when handling data because of the large overall variation. For example, in QGA, two specimens were analyzed for Pb at sizes of ≥ 9.5 mm, one of which was significantly different from the other; in other words, one was 13,700 mg/kg, while the other was 34 mg/kg. For Au, the content of UDGA, UIGA, and UFGA at ≤ 0.5 mm was higher than in QBA, where its content exceeded the quality of general gold ore in plant B. For Ag, the content of any BA and GA was high at particle sizes of ≤ 0.5 mm or 0.5–2.0 mm, while the content of UFGA was more than 10-fold higher than QBA. In plant B, the Pd in UFGA was also more than 10-fold higher than QBA at a particle size of 0.5–2.0 mm. In addition, Zn was 10-fold higher than the QBA in UIGA and UFGA when compared with the 0.5–2.0 mm particle size in plant B. Although the particle size range was slightly different, the Pb in QGA in plant A was lower than that of a previous study, while Zn was similar or slightly lower [18].
Although many metal elements have been reported to have high concentrations in small particle sizes [24,25,26], this was not the case for Cu, Pb, and Zn. Compared with previous studies on municipal solid waste incinerators [23], the Pb, Cu, Zn, Cd, Cr, and Ag contents in QBA contained almost the same magnitude, whereas the contents of Cu and Zn for the 2.0–4.75 mm fraction in plant A were higher than those of previous studies [23]. Additional information on other elements and samples are reported in the Appendix.
Figure 4 shows the element distribution obtained from the particle size accumulation curves in Figs. 2 and 3 and the total content by particle size in Tables 3, 4, 10 and 11 (see Appendix). Figure 4 focuses on the elements listed in the cement acceptance criteria in Table 2 [21]. Among these elements (Tables 3, 4), the data for precious metals, harmful metals, and main base metals exceeded the lower limit of quantification. In plant A, QBA, DDQBA, and QGA showed no significant difference in distribution ratio, whereas, in plant B, GA tended to be distributed in larger particle sizes in QBA and QGA. This is because the particle size distribution was relatively similar (d50 = 1–2 mm) among QBA, DDQBA, and QGA in plant A (Fig. 2), whereas, for QBA and GA (Fig. 3), GA had a larger weight ratio for the large particle sizes (d50 = 5.5 mm).
By element, we confirmed that Cl, SO3, Cd, Cr, and Hg tend to be easily distributed in the small particle size fraction of ≤ 2.0 mm in plant A incineration residue.
Elution test
Table 5 lists the results of the 1M hydrochloric acid extraction test and heavy metal elution test for Pb, Cd, and Cr in incineration residue of plant A.
1 M hydrochloric acid extraction test
The standard value of the 1 M hydrochloric acid extraction test in Japan is 150 mg/kg for both Pb and Cd and 250 mg/kg for Cr (VI). For Pb, QBA and DDQBA at ≤ 4.75 mm and QGA at ≤ 9.5 mm exceeded the standard values. For QBA and QGA, Cd was lower than the standard value, but the overall trend was high for samples with a small particle size of ≤ 2.0 mm. In addition, the hydrochloric acid extraction amount for QGA tended to be higher than that for QBA and DDQBA. In contrast to Pb and Cd, the Cr in QGA was lower than that for QBA and DDQBA. In addition, we confirmed that the elution amount tended to increase with a decrease in the particle size.
Heavy metal elution test
The slag elution standard value was the same as the standard value in the JLT46. The values of Pb and Cd were 10 μg/L, while Cr (VI) was 50 μg/L. Lead exceeded the standard values for most particle sizes and incineration residues. Although the variation in Cr was large, we confirmed that an overall smaller particle size resulted in a larger elution amount. All Cd values were less than the lower limit of quantification (1 μg/L).
Desalination test
Na and K
For both Na and K, the amount of elution on the small particle size fraction was larger, while the amount of elution was higher in DDQBA than in QGA. For K, the elution ratio (= elution amount/total content) was higher for smaller particle sizes in samples collected on the same day (Table 6). This suggests that elution is likely to occur on the particle surface owing to the large surface area. The elution ratio of K was approximately 5% for DDQBA and QBA at ≤ 0.5 mm and approximately 4% for QGA.
For both Na and K, the elution amount increased with an increase in the desalination time. We confirmed that the elution amount converged to a constant concentration. The elution ratio of K was approximately 5% even after 168 h (Table 7). Although salts, such as Na and K, are eluted from the surface in the same manner as other heavy metals, it is possible that there is a limit to the amount of elution from ordinary water washing.
Cl
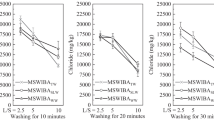
The removal ratio of soluble chlorine was approximately 10–80%, the removal ratio of insoluble chlorine was approximately 0–50%, and the removal ratio of total chlorine was approximately 20–70% (Table 8). The relationship between the size of the diameter and the removal ratio was not confirmed.
With the exception of DDQBA ( ≥ 9.5 mm) and QGA (4.75–9.5 mm), where the total chlorine content was low before desalination, we found that decreasing the content to the acceptance criteria of 1000 mg/kg (= 0.1%) (Table 4), based only on water desalination for all particle sizes, is difficult.
In contrast, with the desalination time (Table 9), the removal ratio of soluble chlorine was approximately 50–70%, the removal ratio of insoluble chlorine was approximately 30–70%, and the removal ratio of total chlorine was approximately 40–70%, suggesting that the elution of chlorine was almost complete in approximately 6 h.
Soluble chlorine appeared to remain after desalination (Tables 8, 9). This may be due to the presence of chlorine compounds that elute at 50 °C, although soluble chlorine is chlorine that can be extracted at 50 °C and does not elute at room temperature (approximately 20 °C) in this experiment.
Quartz (SiO2), calcite (CaCO3), and gehlenite (Ca2Al2SiO7) were detected in almost all of the samples. Friedel’s salt (3CaO Al2O3 CaCl2 10H2O) was detected in DDQBA and QBA at ≤ 0.5, 0.5–2.0, and 2.0–4.75 mm before and after desalination. Although QGA is not shown, the detected mineral and peak intensity were almost identical to DDQBA and QBA. In DDQBA, QBA, and QGA, Friedel’s salt was present in the small particle size fraction before desalination, but the peak intensity decreased after desalination (Figs. 5 and 6).
Based on Friedel’s salt peak intensity for QBA (Fig. 6), some particle sizes were less than half after desalination compared with that before desalination, which is inconsistent with the slight removal ratio of insoluble chlorine (Table 8). One possibility is that this reflects insoluble chlorine, not only in the form of Friedel’s salt but also as new insoluble chlorine, such as AlOCl, as pointed out by Wu et al. [27]. In summary, although the content of Friedel’s salt was less than half, new stable AlOCl was generated, which may have resulted in a slight overall decrease in the insoluble chlorine. However, as AlOCl is close to the diffraction angle of Friedel’s salt and its peak intensity is small, it cannot be identified in this analysis.
If insoluble chlorine decomposes, previous studies have proposed that another mineral forms in its place. Ito et al. [15] suggested that Friedel’s salt (3CaO Al2O3 CaCl2 10H2O) decomposes and produces CaCO3 and Al(OH)3 in the presence of CO2. If this reaction progresses, the peak of CaCO3 should increase after desalination, such that we can detect the peak of CaCl2 · Al (OH)3. However, no such behavior was observed at any particle size, and the cause could not be identified. Portlandite (Ca (OH)2) was detected in the relatively small particle size fraction of DDQBA, QBA, and QGA. Annette et al. [28] reported that oxide lime (CaO) in bottom ash reacts in water to form an alkaline solution and increase pH. Here, we suggest that Ca (OH)2 was generated in the small particle size fraction with a large surface area in DDQBA, QBA, and QGA during the quenching process.
All of the incineration residues showed a high pH exceeding 12 for the small particle size fraction of ≤ 2.0 mm, suggesting that OH– was eluted from Ca(OH)2 (Fig. 7). Therefore, we suggest that the reason that Friedel’s salt exhibited difficulties for decomposition in the small particle size fraction was the high pH.
Conclusions
In this study, we evaluated the resource and environmental safety by clarifying the behavior of the metal concentration, elution, desalination, and insoluble chlorine owing to the differences in the particle sizes of BA and GA. Based on the results, we can draw the following conclusions.
-
(1)
Chlorine, Zn, and Cr exceeded the cement acceptance standard in almost all particle sizes. In plant B, the content of Au, Ag, Pd, and Zn in GA was high for the small particle size fraction of ≤ 2.0 mm compared with QBA. In terms of the element distribution, we confirmed that Cl, SO3, Cd, Cr, and Hg tended to be relatively distributed in the small particle size fraction of ≤ 2.0.
-
(2)
Most particle sizes for Pb in DDQBA, QBA, and QGA exceeded the standard value in the JLT46. Cadmium met the requirements for all particle sizes in QBA, DDQBA, and QGA. For Cr, there was a tendency for smaller particle sizes to result in larger elution amounts.
-
(3)
Both Na and K showed a large amount of elution for small particle sizes. We confirmed that a large surface area resulted in the occurrence of desalination on the particle surface. For the elution amount of the entire sample via time, both Na and K increased with an increase in the desalination time, but K had a desalination ratio of approximately 5% even after 168 h, suggesting that there was a limit to the amount of desalination based only on water washing.
-
(4)
The removal ratio of soluble chlorine by particle size was approximately 10–80%, the removal ratio of insoluble chlorine was approximately 0–50%, and the removal ratio of total chlorine was approximately 20–70%. There was no relationship between the particle size and removal ratio. The chlorine removal ratio by desalination was approximately 50–70% for soluble chlorine, approximately 30–70% for insoluble chlorine, and approximately 40–70% for total chlorine, suggesting that chlorine elution is almost complete in 6 h.
Attempts were made to comprehensively evaluate the resource and environmental safety by analyzing the metal concentration, elution/desalination behavior, and chlorine removal ratio due to particle size differences in BA and GA. We note that the variation in the content data is large, and the element content varies depending on the plant. However, there is little knowledge on the content of GA, elution, and desalination behavior. In particular, from a metal content perspective, it is important to separate and discharge GA with high concentrations of useful and heavy metals from BA in the incineration facility to increase the recycling amount of BA and GA. We expect that our results will be utilized for future incineration residue recycling. Technological developments and cost evaluations of processes that combine the removal of Cl and the recovery/removal of valuable/hazardous metals in BA or GA according to particle size are necessary to promote the recycling of incineration residues in future studies.
References
Ministry of the Environment (2018) Annual report of waste management in Japan, Add Mater p.31
Sarmiento LM, Clavier KA, Paris JM, Ferraro CC, Townsend TG (2019) Critical examination of recycled municipal solid waste incineration ash as a mineral source for portland cement manufacture – A case study. Resour Conserv Recycl 148:1–10
Lynn CJ, Ghataora GS, Dhir OBERK (2017) Municipal incinerated bottom ash (MIBA) characteristics and potential for use in road pavements. Int J Pavement Res Technol 10:185–201. https://doi.org/10.1016/j.ijprt.2016.12.003
Hyks J, Astrup T (2009) Influence of operational conditions, waste input and ageing on contaminant leaching from waste incinerator bottom ash: a full-scale study. Chemosphere 76:1178–1184. https://doi.org/10.1016/j.chemosphere.2009.06.040
Freyssinet P, Piantone P, Azaroual M, Itard Y, Clozel-Leloup B, Guyonnet D, Baubron JC (2002) Chemical changes and leachate mass balance of municipal solid waste bottom ash submitted to weathering. Waste Manag 22:159–172. https://doi.org/10.1016/S0956-053X(01)00065-4
Rem PC, De Vries C, Van Kooy LA, Bevilacqua P, Reuter MA (2004) The Amsterdam pilot on bottom ash. Miner Eng 17:363–365. https://doi.org/10.1016/j.mineng.2003.11.009
Holm O, Thomé-Kozmiensky E (2018) Removal, treatment and utilization of waste incineration bottom ash
Bunge R (2019) Recovery of metals from waste incinerator bottom ash, UMTEC https://www.umtec.ch/fileadmin/user_upload/umtec.hsr.ch/Dokumente/Metals_from_MWIBA_6_2019.pdf
Holm O, Simon FG (2017) Innovative treatment trains of bottom ash (BA) from municipal solid waste incineration (MSWI) in Germany. Waste Manag 59:229–236. https://doi.org/10.1016/j.wasman.2016.09.004
Xuan D, Tang P, Poon CS (2018) Limitations and quality upgrading techniques for utilization of MSW incineration bottom ash in engineering applications – A review. Constr Build Mater 190:1091–1102. https://doi.org/10.1016/j.conbuildmat.2018.09.174
Taiheiyo cement corporation website. https://www.taiheiyo-cement.co.jp/english/service_product/recycle_mw/hai/index.html Accessed 15 Jun 2020
Suryavanshi AK, Scantlebury JD, Lyon SB (1996) Mechanism of Friedel’s salt formation in cements rich in tri-calcium aluminate
Suryavanshi AK, Narayan Swamy R (1996) Stability of Friedel’s salt in carbonated concrete structural elements. Cem Concr Res 26:729–741. https://doi.org/10.1016/S0008-8846(96)85010-1
Rapin JP, Renaudin G, Elkaim E, Francois M (2002) Structural transition of Friedel’s salt 3CaO·Al2O3·CaCl2·10H2O studied by synchrotron powder diffraction. Cem. Concr. Res. 32:513–519
Ito R, Dodbiba G, Fujita T, Ahn JW (2008) Removal of insoluble chloride from bottom ash for recycling. Waste Manag 28:1317–1323. https://doi.org/10.1016/j.wasman.2007.05.015
Xia Y, He P, Shao L, Zhang H (2017) Metal distribution characteristic of MSWI bottom ash in view of metal recovery. J Environ Sci (China) 52:178–189. https://doi.org/10.1016/j.jes.2016.04.016
Allegrini E, Maresca A, Olsson ME, Holtze MS, Boldrin A, Astrup TF (2014) Quantification of the resource recovery potential of municipal solid waste incineration bottom ashes. Waste Manag 34:1627–1636. https://doi.org/10.1016/j.wasman.2014.05.003
Inkaew K, Saffarzadeh A, Shimaoka T (2014) Characterization of grate sifting deposition ash, unquenched bottom ash and water-quenched bottom ash from mass-burn moving grate waste to energy plant. J Japan Soc Civ Eng Ser G (Environmental Res 70:III_469-III_475. https://doi.org/https://doi.org/10.2208/jscejer.70.iii_46919.
Wiles CC (1996) Municipal solid waste combustion ash: State-of-the-knowledge. J Hazard Mater 47:325–344. https://doi.org/10.1016/0304-3894(95)00120-4
Yamamoto H, Yokoyama T, Oshita K, Takaoka M, Takeda N (2007) The study of shifting of valuable metals to riddling ash in municipal solid waste incineration process (in Japanese). J Japan Soc Waste Manag Expert 18:314–324. https://doi.org/10.3985/jswme.18.314
Ministry of Economy, Trade and Industry (2004) Survey report on domestic wide area treatment system construction of municipal waste incineration ash (in Japanese) 63, 65 https://www.meti.go.jp/policy/recycle/main/data/research/pdf/150711-3_jilc_7.pdf (in Japanese) Accessed 24 Nov 2019
Japan society of Material Cycles and Waste Management website, Provisional analysis method for rare metals in products (in Japanese) (2010) https://jsmcwm.or.jp/materialflow/files/2010/11/methodv2_20101217.doc Accessed 15 Jun 2020
Lin WY, Heng KS, Sun X, Wang JY (2015) Accelerated carbonation of different size fractions of MSW IBA and the effect on leaching. Waste Manag 41:75–84. https://doi.org/10.1016/j.wasman.2015.04.00324
del Valle-Zermeño R, Gómez-Manrique J, Giro-Paloma J, Formosa J, Chimenos JM (2017) Material characterization of the MSWI bottom ash as a function of particle size. Effects of glass recycling over time. Sci Total Environ 581–582:897–905. https://doi.org/10.1016/j.scitotenv.2017.01.047
Pérez-Martínez S, Giro-Paloma J, Maldonado-Alameda A, Formosa J, Queralt I, Chimenos JM (2019) Characterisation and partition of valuable metals from WEEE in weathered municipal solid waste incineration bottom ash, with a view to recovering. J Clean Prod 218:61–68. https://doi.org/10.1016/j.jclepro.2019.01.313
Maldonado-Alameda A, Giro-Paloma J, Svobodova-Sedlackova A, Formosa J, Chimenos JM (2020) Municipal solid waste incineration bottom ash as alkali-activated cement precursor depending on particle size. J Clean Prod. https://doi.org/10.1016/j.jclepro.2019.118443
Wu B, Wang D, Chai X, Takahashi F, Shimaoka T (2016) Characterization of chlorine and heavy metals for the potential recycling of bottom ash from municipal solid waste incinerators as cement additives. Front Environ Sci Eng. https://doi.org/10.1007/s11783-016-0847-9
Johnson CA, Brandenberger S, Baccini P (1995) Acid neutralizing capacity of municipal waste incinerator bottom ash. Environ Sci Technol 29:142–147. https://doi.org/10.1021/es00001a018
Acknowledgments
We appreciate the local governments for their cooperation in providing incineration bottom and grate sifting deposition ash samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iino, S., Tatsuichi, S. & Miyawaki, K. Characterization of metal concentration, heavy metal elution, and desalination behavior of municipal solid waste incineration bottom and grate sifting deposition ash based on particle size. J Mater Cycles Waste Manag 23, 341–357 (2021). https://doi.org/10.1007/s10163-020-01136-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-020-01136-w